Article
A stain on iron therapy
- Martin Canning, Louise Grannell
- Aust Prescr 2020;43:160-3
- 1 October 2020
- DOI: 10.18773/austprescr.2020.051
Iron staining is an unwanted and in some cases permanent adverse effect of intravenous iron administration. Cosmetically unacceptable staining may cause distress and have psychological implications for the patient.
There should be a suitable indication for parenteral iron therapy. Patients must be advised of the risk of harm and give their informed consent before receiving parenteral iron.
Strategies to minimise the risks of staining with intravenous iron include appropriate cannulation and close monitoring of the infusion. Stop the infusion if there are signs of extravasation.
Laser therapy may be a treatment option in cases of persistent discolouration due to iron staining.
Iron deficiency is a common condition and a large contributor to anaemia.1 The prevalence of iron deficiency anaemia is high in younger women and indigenous Australians.2 Treatment options to correct iron deficiency in Australia include oral and parenteral iron.3 Within the last decade the use of intravenous iron has been increasing,4 particularly in the community. This is because of newer iron salts with favourable adverse effect profiles and shorter infusion times for intravenous formulations. These include ferric carboxymaltose and ferric derisomaltose. For patients in hospital, iron polymaltose or iron sucrose can also be used.
An uncommon adverse effect of parenteral iron is skin staining (see Fig.). This is not a new phenomenon as it is a well-known adverse effect of intramuscular iron.5 Iron staining can occur with intravenous infusions if there is extravasation into the surrounding tissue. The use of intramuscular iron administration is limited in practice,3 but the injection can be given into an unexposed site. However, administration at an unexposed site is not necessarily possible when giving iron intravenously. A rise in reports of iron staining6-10 may correspond with the increasing use of intravenous iron in clinical practice.6-13
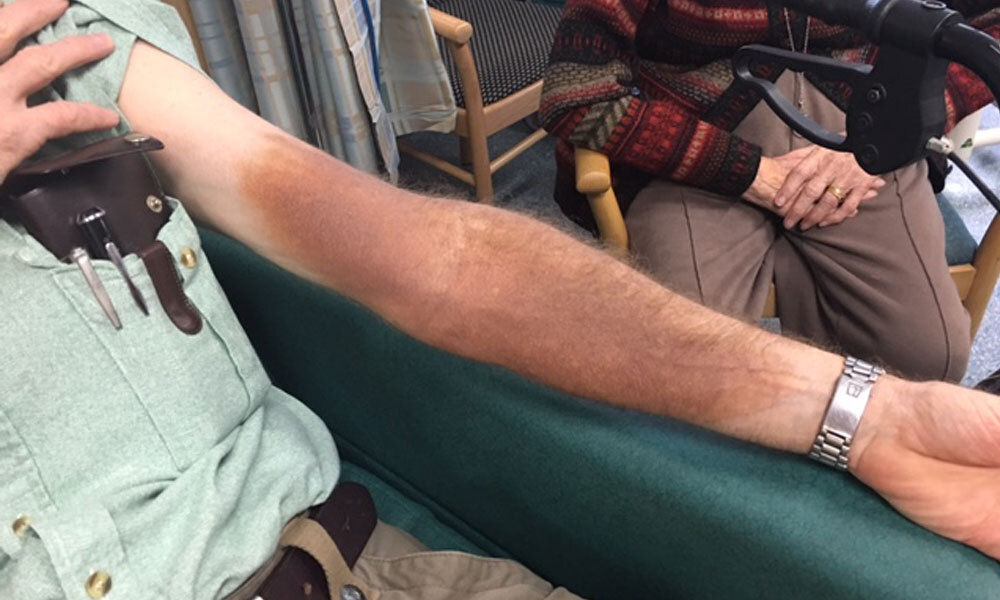
The rate of skin discolouration with intravenous iron preparations has been reported in clinical trials as 0.68%14 to 1.3%.15 Postmarketing reports suggest the incidence may be lower and skin necrosis has not been reported. However, iron staining may be under-reported to pharmacovigilance databases. A review of the French pharmacovigilance database from 2000 to 2016 found only 51 cases of cutaneous pigmentation with iron.12
Postmarketing reports to the Therapeutic Goods Administration (TGA) Database of Adverse Event Notifications,16 from March 2014 to October 2019, included 27 cases for ferric carboxymaltose. These reports included the terms skin discolouration or hyperpigmentation, haemosiderin stain, pigmentation disorder, infusion/injection/administration site discolouration, or extravasation. The TGA data include eight cases of pigmentation disorder or skin discolouration with iron polymaltose, with the first report in 2005. There are currently no reports for ferric derisomaltose, but this adverse effect is included in the product information.
Box 1 - Principles for minimising the risk of intravenous iron stains
Ensure an appropriate indication for parenteral iron
Inform the patient of the risk of skin staining at the initial consultation
Ensure the correct injection site and administration technique is used
Monitor closely for signs and symptoms of extravasation
Box 2 - Infusion technique to minimise the risk
of iron staining
Avoid intravenous iron administration via cannulation at sites of flexion (e.g. antecubital fossa, wrist) or on the back of the hand
The distal veins of the forearm are the preferred site Use an appropriate cannula size (20- to 24-gauge)
Secure the cannula and use an extension set to minimise catheter movement
Do not cover the injection site with a bandage Minimise the number of cannulation attempts
Ensure the patency of the vein before administration. If patency is uncertain, do not administer intravenous iron
Do not give infusions at night-time
Do not give infusions to patients unable to report symptoms (e.g. anaesthetised)
Once iron deficiency is diagnosed, establish the cause. The decision on appropriate treatment should then consider the patient’s treatment goals. This includes assessing the options for correcting the iron deficiency and their potential adverse effects. Dietary intake, oral supplements or parenteral iron are suitable options.3
Parenteral iron is usually only indicated when oral iron therapy has failed.3 However, there are some patient cohorts who may benefit from intravenous iron without a trial of oral therapy. They include patients who have heart failure with a reduced ejection fraction,15 those undergoing haemodialysis,17 and pregnant women in their second or third trimester requiring rapid iron replenishment.18
Although the incidence of iron staining appears to be relatively low, its potential irreversibility and the cosmetic impact it may have warrant discussion with patients. The Medical Board of Australia has reminded medical practitioners to advise patients about the risk so that they can give informed consent to treatment.19 Using a patient information brochure about iron staining may assist with this. The BloodSafe organisation has a useful leaflet available in English and other languages.20 When intravenous iron is indicated and patients choose to receive an infusion, it is advisable to document the content and outcome of the discussion about risks including discolouration or staining.
The infusion sites used for intravenous therapy may influence the rate of extravasation due to the potential for vessel damage related to movement of the cannula.21,22 Administration of intravenous iron via cannulation at sites of flexion (e.g. antecubital fossa, wrist) or on the back of the hand should be avoided when possible. If these sites must be used, the smallest suitable cannula size may reduce the likelihood of vessel trauma.22 Try to minimise catheter movement by securing the cannula21-23 and using an extension set.24 When using smaller gauge devices, it may be necessary to slow the infusion to minimise the risk of dislodgement.25
The number of attempts at cannulation should be minimised as there is an increased risk of extravasation due to multiple venous punctures.21,22 For patients who are difficult to cannulate, seek the expertise of more experienced staff. Although postponing intravenous iron therapy may inconvenience the patient, it is unlikely to result in adverse clinical outcomes. Intravenous iron infusion is rarely urgent.
The patency of the cannula should be checked by giving 5–10 mL of sodium chloride 0.9% before the infusion.21
The review of cutaneous pigmentation reported to the
French pharmacovigilance database suggested
improvements in monitoring are necessary to detect
extravasation.12
Patients who experience iron extravasation resulting
in staining may describe pain, swelling, and
feelings of pressure or pricking at the infusion
site.13 Patients
should therefore be told to notify staff of any of
these symptoms (Box 3). This is an
important consideration for patients who do not
understand English. Administration of intravenous
iron must be avoided if the patient’s ability to
report these symptoms is reduced (e.g. anaesthetised
patients). Early cessation of the infusion may limit
the amount of solution that enters the tissues and
could minimise the extent of staining.
Box 3 - Clinical features of iron extravasation6-13
Symptoms during
infusion
Pain, swelling,
feeling of pressure, prickling on the
injection site and immediately observable
staining. Note: some patients report no pain
or other symptoms during the infusion and
the discolouration appears hours or days
later
Extent of skin
discolouration
Can be
localised to around the injection site or
extend along the length of the arm. May be
patchy or consistent discolouration
Colour changes
Most
common – light to dark brown
Less common
– black, bluish, purple, grey
Symptoms in the longer
term
Generally,
discolouration is asymptomatic, but some
patients complain of aching, changed
sensitivity in the affected area or
tenderness on palpation
Outcome
In many cases,
iron staining is permanent. Some patients
report fading of the stain over time or
successful treatment with laser therapy
Close assessment of the cannula site during infusion is essential to enable early identification of extravasation. The site should never be covered up with a bandage. Observations of the cannula site should be timed to correspond with monitoring of the patient’s other vital signs in accordance with local protocols for infusions.26 Giving intravenous iron infusions overnight must be avoided as it is more difficult to observe extravasation and staining in the dark.
In order to ensure the best outcomes for patients, health professionals involved with the prescribing, administration and monitoring of intravenous iron must be adequately trained and competent. A set protocol that outlines best practice for intravenous iron administration, including cannulation, should be followed. Staff must be aware of the monitoring requirements and the symptoms of potential adverse effects.
There are no published guidelines outlining how to manage iron extravasation or skin discolouration following iron infusions. Box 4 gives the best available guidance for acute management to limit the potential for further staining. Clinical photographs should also be taken to capture the extent of the extravasation and to help with monitoring the success of subsequent treatments.
Box 4 - Acute management of iron extravasation
If the patient complains of pain, swelling, soreness at the injection site or there is any obvious swelling or discolouration, stop the infusion immediately and assess the site
Disconnect the giving set
Aspirate any residual drug from the cannula
Remove the cannula
Apply a cold pack if there is swelling or soreness, however this does not appear to prevent the spread of the stain
There are limited options to reverse iron staining. Topical therapies, lymphatic drainage and massages have been tried without success.9,13 The most evidence for successful reversal of iron staining is with laser therapy.
One review assessed 29 patients who had reported accidental staining from iron infusions over a nine-year period.13 Thirteen patients had laser therapy and eight completed treatment. Regression of iron staining took an average of 5.6 laser sessions over one to two years. The type of laser is important with most evidence being for quality-switched Nd:YAG or picosecond. The patient’s individual skin type may also influence the success of laser treatment. In general, laser therapy was well tolerated.
Laser therapy is available in Australia, but there may be significant financial barriers as repeated applications are required. If the patient is concerned about the staining, early referral to a dermatologist with a laser clinic specialising in quality-switched Nd:YAG and picosecond laser is appropriate.
When extravasation occurs, prudent review of the patient is warranted. Consider likely contributing factors, such as whether there was a suitable indication for intravenous iron, poor techniques in cannulation, the patient’s own vasculature and any lack of monitoring. Report these cases to the TGA.
There should be a clear indication for using intravenous iron. Patients need to give informed consent for the infusion.
Iron extravasation can be cosmetically unacceptable for patients so strategies should be put in place to prevent it from occurring. These include appropriate vein selection, securing the cannula and close monitoring during the infusion. In addition, the patient should be advised to report any pain, irritation or swelling at the infusion site.
In the event of extravasation and persistent staining, repeated laser sessions over one to two years may be required. However, iron staining can be permanent.
Conflict of interest: none declared
Acknowledgment: A thank you is extended to Carmela Corallo, Formulary Manager at Alfred Health, for her translation skills and review of this manuscript and Jana Waldmann, Librarian, The Prince Charles Hospital Library, who assisted by performing a literature search.
Australian Prescriber welcomes Feedback.
Hollands L. PBS/RPBS prescriptions for ATC4 B03AC – Iron, parenteral preparations, supplied between 1 January 2006 to 31 December 2018. Email from [email protected], 2019 Dec 3.

Safety and quality pharmacist, The Prince Charles Hospital, Metro North Hospital and Health Service, Brisbane
Senior medicines information pharmacist, Alfred Health, Melbourne