SUMMARY CMI
ABILIFY™
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using ABILIFY?
ABILIFY contains the active ingredient aripiprazole. ABILIFY is used to treat symptoms of schizophrenia. ABILIFY may be given to treat acute episodes of sustained upward mood swings (mania) in adult patients with Bipolar 1 Disorder. During mania, patients experience episodes of overactivity, elation or irritability.
For more information, see Section 1. Why am I using ABILIFY? in the full CMI.
2. What should I know before I use ABILIFY?
Do not use if you have ever had an allergic reaction to ABILIFY or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use ABILIFY? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with ABILIFY and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use ABILIFY?
- Unless your doctor gives you other directions, you should take ABILIFY only once a day.
- Take ABILIFY at about the same time each day.
More instructions can be found in Section 4. How do I use ABILIFY? in the full CMI.
5. What should I know while using ABILIFY?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using ABILIFY? in the full CMI.
6. Are there any side effects?
Common side effects are headache; indigestion; nausea; vomiting; insomnia; constipation; light-headedness; drowsiness; agitation; anxiety; inability to sit or stand still, restless movement of the arms and legs. Serious side effects include seizure; fits or convulsions; fainting; sudden increase in body temperature; sweating.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
ABILIFY™
Active ingredient(s): Aripiprazole (Ari-pip-rah-zol)
Consumer Medicine Information (CMI)
This leaflet provides important information about using ABILIFY. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using ABILIFY.
Where to find information in this leaflet:
1. Why am I using ABILIFY?
2. What should I know before I use ABILIFY?
3. What if I am taking other medicines?
4. How do I use ABILIFY?
5. What should I know while using ABILIFY?
6. Are there any side effects?
7. Product details
1. Why am I using ABILIFY?
ABILIFY contains the active ingredient aripiprazole. ABILIFY belongs to a group of medicines called antipsychotic agents which improve the symptoms of certain types of mental illness.
ABILIFY is used to treat symptoms of schizophrenia.
ABILIFY may be given to treat acute episodes of sustained upward mood swings (mania) in adult patients with Bipolar 1 Disorder. During mania, patients experience episodes of overactivity, elation or irritability.
Schizophrenia is a mental illness with disturbances in thinking, feelings and behaviour.
Bipolar disorder is a condition with symptoms such as feeling "high", having excessive amounts of energy, needing much less sleep than usual, talking very quickly with racing ideas and sometimes severe irritability.
Your doctor may have prescribed ABILIFY for another reason. Ask your doctor if you have any questions about why ABILIFY has been prescribed for you.
There is no evidence that ABILIFY is addictive.
This medicine is available only with a doctor's prescription.
ABILIFY is not recommended for use in children under the age of 18, as safety and efficacy have not been established in this age group.
2. What should I know before I use ABILIFY?
Warnings
Do not use ABILIFY if:
- you are allergic to aripiprazole, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
Symptoms of an allergic reaction include:
- rash, itching or hives on the skin
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
Tell your doctor or pharmacist if you have allergies to:
- any other medicines
- any other substances such as foods, preservatives or dyes
Do not take ABILIFY after the expiry or use by date printed on the pack.
If you take this medicine after this date has passed, it may not work as well.
Do not take ABILIFY if the packaging is torn or shows signs of tampering.
If this is the case, return it to your pharmacist.
If you are not sure whether you should start taking ABILIFY, talk to your doctor or pharmacist.
Check with your doctor if you:
- have any other medical conditions especially the following:
- a reaction to some medicines with a sudden increase in body temperature, sweating, fast heartbeat, muscle stiffness and fluctuating blood pressure, which may lead to coma. This reaction is called neuroleptic malignant syndrome.
- a reaction to some medicines with abnormal movements of the tongue, or other uncontrolled movements of the mouth, tongue, cheeks or jaw which may progress to the arms and legs. This reaction is called tardive dyskinesia.
- low blood pressure
- problems with your heart or blood vessels
- epilepsy, seizures or fits
- problems with your oesophagus (food pipe) such as difficulty in swallowing
- high blood sugar or diabetes mellitus
- Alzheimer's disease or dementia
- alcohol or drug abuse or dependence or a history of one of these
- venous thromboembolism or are at risk of venous thromboembolism
- have a history of or are at risk of sleep apnea (a sleep disorder where your breathing is interrupted during sleep)
- lactose intolerance - take any medicines for any other condition
Tell your doctor if you have past experience of excessive gambling. Tell your doctor if you or your family/carer notices that you are developing urges or cravings to behave in ways that are unusual for you and you cannot resist the impulse, drive or temptation to carry out certain activities that could harm yourself or others. These are called impulse control disorders and can include behaviours such as addictive gambling, excessive eating or spending, an abnormally high sex drive or preoccupation with an increase in sexual thoughts or feelings.
Your doctor may need to adjust or stop your dose.
All thoughts of suicide must be taken seriously. Tell your doctor or a mental health professional immediately if you have any suicidal thoughts, feelings about hurting yourself or other significant mood changes.
Suicidal thoughts and behaviours have been reported during ABILIFY treatment.
Tell your doctor if you drink alcohol.
Your doctor may advise you to avoid alcohol as it can magnify the side-effects of this medicine.
Aripiprazole may cause sleepiness, fall in blood pressure when standing up, dizziness and changes in your ability to move and balance, which may lead to falls. Caution should be taken.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking ABILIFY.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
ABILIFY is not recommended for use during pregnancy. If you need to take ABILIFY during your pregnancy, your doctor will discuss with you the benefits and risks of taking it. Babies exposed to antipsychotics (including ABILIFY) during the third trimester of pregnancy are at risk of experiencing shaking, muscle stiffness, difficulty in feeding and/or withdrawal symptoms. These symptoms may resolve spontaneously or require additional medical treatment.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
It is recommended that you do not breast-feed while taking ABILIFY, as it may pass into breast milk and therefore there is a possibility that the breast-fed baby may be affected.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with ABILIFY and affect how it works. These include:
- medicines used to treat brain disorders such as, anxiety, depression, mood swings, epilepsy or seizures, Parkinson's disease or insomnia
- medicines used to treat high blood pressure
- medicines used to treat fungal infections
- medicines used to treat heart rhythm disturbances
- medicines used to treat bacterial or viral infections
- a medicine called cyclosporin (Neoral®; Sandimmun®)
- a medicine called cimetidine (Tagamet®; Magicul®)
These medicines may be affected by ABILIFY, or may affect how well it works. Your doctor may need to adjust your dose of Abilify or of the other medicine.
Eating grapefruit or drinking grapefruit juice may affect how ABILIFY works.
Your doctor or pharmacist may have more information on medicines to be careful with or avoid while taking ABILIFY.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect ABILIFY.
4. How do I use ABILIFY?
How much to take / use
- Unless your doctor gives you other directions, you should take ABILIFY only once a day.
- ABILIFY tablets should be swallowed whole and washed down with a glass of water.
- Follow the instructions provided and use ABILIFY until your doctor tells you to stop.
- ABILIFY helps to control your condition but does not cure it. Therefore you must take ABILIFY every day. Improvement in symptoms may take several days to some weeks to occur. Even if you feel better do not stop taking ABILIFY unless your doctor tells you to.
When to take / use ABILIFY
- Take ABILIFY at about the same time each day.
Taking the medicine at the same time each day will have the best effect. It will also help you remember when to take it. - It does not matter whether you take ABILIFY with or without food.
If you forget to use ABILIFY
ABILIFY should be used regularly at the same time each day. If you miss your dose at the usual time, follow the instructions below:
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you use too much ABILIFY
If you think that you have used too much ABILIFY, you may need urgent medical attention.
Patients who have taken too much ABILIFY have experienced the following symptoms:
- drowsiness or sleepiness,
- high blood pressure
- rapid heartbeat
- vomiting
- reduced level of consciousness
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using ABILIFY?
Things you should do
- If you are about to be started on any new medicine, tell your doctor, dentist and pharmacist that you are taking ABILIFY.
- If you plan to have any kind of surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking ABILIFY.
- Be sure to keep all of your doctor's appointments so that your progress can be checked.
Call your doctor straight away if you:
- Become pregnant while taking ABILIFY
Remind any doctor, dentist or pharmacist you visit that you are using ABILIFY.
Things you should not do
- Do not give ABILIFY to anyone else, even if their symptoms seem similar or they have the same condition as you.
- Do not take ABILIFY to treat any other complaints unless your doctor or pharmacist tells you to.
- Do not stop taking ABILIFY or lower the dosage, even if you are feeling better, without checking with your doctor. If you stop taking ABILIFY suddenly your condition may worsen.
- Do not take more of this medicine and do not take it more often than your doctor has ordered.
Make sure you keep cool in hot weather and keep warm in cool weather.
ABILIFY may affect the way your body reacts to temperature changes. It may prevent sweating, even during heatwaves. You may feel dizzy or faint if you are too hot. To stay cool in hot weather, try to do the following:
- wear light clothing
- spend time in air-conditioned environments (or keep windows open and use electric fans)
- drink plenty of water
- take cool baths or showers and avoid hot baths and saunas
- try to restrict exercise or heavy work to cool parts of the day
Driving or using machines
Make sure that you know how you react to ABILIFY before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy or light headed or not alert.
ABILIFY may cause some people to become drowsy or less alert than they are normally or cause light-headedness, dizziness or tiredness. If this occurs do not undertake the activity.
If ABILIFY makes you feel light-headed, dizzy or faint, be careful when getting up from a sitting or lying position.
Getting up slowly may help.
Drinking alcohol
Tell your doctor if you drink alcohol.
Be careful when drinking alcohol while taking ABILIFY.
Your doctor may suggest you avoid alcohol while you are being treated with ABILIFY.
Looking after your medicine
Store ABILIFY in a cool place where the temperature stays below 30°C.
Heat and dampness can destroy some medicines
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines. Do not keep outdated medicine or medicine no longer needed.
When to discard your medicine
If your doctor tells you to stop taking ABILIFY or the medicine has passed its expiry date, ask your pharmacist what to do with any leftover medicine.
Be sure that any discarded medicine is out of the reach of children.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
ABILIFY is generally well-tolerated and the side effects are often hard to distinguish from disease symptoms. It is important that you tell your doctor as soon as possible about any unwanted effects.
Less serious side effects
| Less serious side effects | What to do |
Gastrointestinal related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Pain related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor if you have obsessive (recurring) thoughts or behaviours or trouble controlling impulsive urges or while taking ABILIFY.
Obsessive compulsive behaviours (feeling the need to check things repeatedly or having certain thoughts repeatedly), gambling urges, sexual urges, compulsive spending, binge or compulsive eating and other urges have occurred in some patients.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Also, while taking ABILIFY, some elderly patients with dementia have suffered serious side effects such as a "mini" stroke, stroke, pneumonia or heart problems. These serious side effects can be life threatening.
Do not be alarmed by this list of possible side effects.
You may not experience any or only some of them.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What ABILIFY contains
| Active ingredient (main ingredient) | aripiprazole |
| Other ingredients (inactive ingredients) | lactose, starch - maize, cellulose - microcrystalline, hydroxypropylcellulose and magnesium stearate. The following colorants are also present in the tablets:
|
| Potential allergens | ABILIFY does not contain sucrose, gluten, tartrazine or any other azo dyes. |
Do not take this medicine if you are allergic to any of these ingredients.
What ABILIFY looks like
5mg ABILIFY tablets are blue, rectangular with a bevel edge and are marked on one side with "A-007" and "5" (Not available in New Zealand).
(Aust R 90925)
10mg ABILIFY tablets are pink, rectangular with a bevel edge and are marked on one side with "A-008" and "10".
(Aust R 90997)
15mg ABILIFY tablets are yellow, round with a bevel edge and are marked on one side with "A-009" and "15". (Aust R 90998)
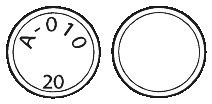
20mg ABILIFY tablets are white to pale yellowish white, round with a bevel edge and are marked on one side with "A-010" and "20".
(Aust R 90999)
30mg ABILIFY tablets are pink, round with a bevel edge and are marked on one side with "A-011" and "30".
(Aust R 91000)
ABILIFY tablets are packed in aluminium blisters in a carton. Each carton contains 30 tablets.
Who distributes ABILIFY
In Australia:
Otsuka Australia Pharmaceutical Pty Ltd
Suite 2.03, Level 2
9 Help Street
Chatswood NSW 2067
Tel: Toll Free 1800 059 606
In New Zealand:
Pharmacy Retailing (NZ) Ltd
trading as Healthcare Logistics
58 Richard Pearse Drive
Airport Oaks
Mangere
Auckland 2022
Tel: Toll free 0800 602 200
This leaflet was prepared in October 2022.
ABILIFY is a trademark of Otsuka Pharmaceutical Co., Ltd.
Published by MIMS December 2022






 An examination of population subgroups did not reveal any clear evidence of differential adverse reaction incidence on the basis of age, gender, or race.
An examination of population subgroups did not reveal any clear evidence of differential adverse reaction incidence on the basis of age, gender, or race.

 A 52 week, haloperidol controlled, long-term, maintenance trial (n = 1294) was conducted in patients with acute relapse of chronic schizophrenia. In this trial involving the administration of Abilify 30 mg/day and haloperidol 10 mg/day, with a one time option to decrease Abilify to 20 mg/day and haloperidol to 7 mg/day, Abilify was at least comparable to haloperidol in time to failure to maintain response in responders. Based on patients who responded at any time during the 52 week study (610/853, 72% in the Abilify group and 298/430, 69% in the haloperidol group), there was a 12% lower risk of subsequent failure with Abilify relative to haloperidol (relative risk: 0.881, 95% CI: 0.645 to 1.204). Abilify was comparable to haloperidol in time to failure to maintain response in all randomised patients. Patients in the Abilify group had a 14% lower risk of failure compared with the haloperidol group (relative risk: 0.858, 95% CI: 0.721, 1.021). Abilify was statistically superior to haloperidol in the analysis of the proportion of patients on treatment and in response at weeks 8, 26 and 52 (prespecified key time points). At week 52, 40% of Abilify patients were still on study and in response compared to 27% of haloperidol patients (p < 0.001). Abilify treated patients had a statistically significant lower risk (31%) of discontinuations due to lack of efficacy or adverse event relative to haloperidol treated patients (relative risk 0.692; 95% CI: 0.573 to 0.837). There were no significant differences between Abilify and haloperidol groups in terms of change from baseline PANSS total scores, PANSS positive subscores, CGI severity or improvement scores. Abilify did result in a significantly greater improvement in the PANSS negative subscores at weeks 26 and 52 and the MADRS total score at weeks 8, 26 and 52. [Mean change PANSS negative subscale score (week 26: p = 0.029; 95% CI: -1.52, -0.08) (week 52: p = 0.011; 95% CI: -1.73, -0.23). Mean change MADRS total score (week 8: p = 0.027; 95% CI: -1.74, -0.11) (week 26: p = 0.22; 95% CI: -1.95, -0.15) (week 52: p = 0.031; 95% CI: -1.97, -0.09)].
A 52 week, haloperidol controlled, long-term, maintenance trial (n = 1294) was conducted in patients with acute relapse of chronic schizophrenia. In this trial involving the administration of Abilify 30 mg/day and haloperidol 10 mg/day, with a one time option to decrease Abilify to 20 mg/day and haloperidol to 7 mg/day, Abilify was at least comparable to haloperidol in time to failure to maintain response in responders. Based on patients who responded at any time during the 52 week study (610/853, 72% in the Abilify group and 298/430, 69% in the haloperidol group), there was a 12% lower risk of subsequent failure with Abilify relative to haloperidol (relative risk: 0.881, 95% CI: 0.645 to 1.204). Abilify was comparable to haloperidol in time to failure to maintain response in all randomised patients. Patients in the Abilify group had a 14% lower risk of failure compared with the haloperidol group (relative risk: 0.858, 95% CI: 0.721, 1.021). Abilify was statistically superior to haloperidol in the analysis of the proportion of patients on treatment and in response at weeks 8, 26 and 52 (prespecified key time points). At week 52, 40% of Abilify patients were still on study and in response compared to 27% of haloperidol patients (p < 0.001). Abilify treated patients had a statistically significant lower risk (31%) of discontinuations due to lack of efficacy or adverse event relative to haloperidol treated patients (relative risk 0.692; 95% CI: 0.573 to 0.837). There were no significant differences between Abilify and haloperidol groups in terms of change from baseline PANSS total scores, PANSS positive subscores, CGI severity or improvement scores. Abilify did result in a significantly greater improvement in the PANSS negative subscores at weeks 26 and 52 and the MADRS total score at weeks 8, 26 and 52. [Mean change PANSS negative subscale score (week 26: p = 0.029; 95% CI: -1.52, -0.08) (week 52: p = 0.011; 95% CI: -1.73, -0.23). Mean change MADRS total score (week 8: p = 0.027; 95% CI: -1.74, -0.11) (week 26: p = 0.22; 95% CI: -1.95, -0.15) (week 52: p = 0.031; 95% CI: -1.97, -0.09)].
