SUMMARY CMI
ALEPAM
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using ALEPAM?
ALEPAM contains the active ingredient oxazepam. ALEPAM is used to anxiety, tremor, confusion or anxiety associated with alcohol withdrawal.
For more information, see Section 1. Why am I using ALEPAM? in the full CMI.
2. What should I know before I use ALEPAM?
Do not use if you have ever had an allergic reaction to ALEPAM or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use ALEPAM? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with ALEPAM and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use ALEPAM?
- The dose will vary between patients. Your doctor will tell you how many tablets you need to take each day and when to take them.
- Swallow ALEPAM with a glass of water, with or without food.
- Take ALEPAM only for as long as your doctor recommends. It is usually used for short periods only (such as 2 to 4 weeks).
More instructions can be found in Section 4. How do I use ALEPAM? in the full CMI.
5. What should I know while using ALEPAM?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using ALEPAM? in the full CMI.
6. Are there any side effects?
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ALEPAM. Some mild side effect include dizziness, drowsiness, feeling tired, lightheadedness or feeling faint and headache. Some serious side effects include confusion, behavioural or mood changes such as sudden outbursts of anger and increased excitement and hallucinations.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
ALEPAM
Active ingredient(s): oxazepam
Consumer Medicine Information (CMI)
This leaflet provides important information about using ALEPAM. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using ALEPAM.
Where to find information in this leaflet:
1. Why am I using ALEPAM?
2. What should I know before I use ALEPAM?
3. What if I am taking other medicines?
4. How do I use ALEPAM?
5. What should I know while using ALEPAM?
6. Are there any side effects?
7. Product details
1. Why am I using ALEPAM?
ALEPAM contains the active ingredient oxazepam. ALEPAM is benzodiazepine which is thought to work by acting on the brain chemicals.
ALEPAM is used to treat:
- anxiety, such as the anxiety associated with depression
- tremor, anxiety and confusion associated with alcohol withdrawal.
Your doctor may have prescribed ALEPAM for another reason. Ask your doctor if you have any questions about why ALEPAM has been prescribed for you.
2. What should I know before I use ALEPAM?
In general, benzodiazepines such as ALEPAM should be taken for short periods only (for example 2 to 4 weeks). Continuous long term use is not recommended unless advised by your doctor. The use of benzodiazepines may lead to dependence on the medicine.
Warnings
Do not use ALEPAM if:
- you are allergic to oxazepam or any other benzodiazepine medicine or any of the ingredients listed at the end of this leaflet.
- you have severe and chronic respiratory (lung or airways) disease.
- you have sleep apnoea.
- the expiry date (EXP) printed on the bottle has passed. If you take this medicine after the expiry date, it may not work as well.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing; wheezing or shortness of breath.
Check with your doctor if you:
- are allergic to any other medicines, foods, dyes or preservatives.
- take any medicines for any other condition.
- have any medical conditions, especially the following:
- low blood pressure
- myasthenia gravis, a condition where there is severe muscle weakness
- glaucoma (increased pressure in the eye)
- liver or kidney problems
- depression, psychosis or schizophrenia
- epilepsy, fits or convulsions
- drug or alcohol dependence or a past history of these problems. - are pregnant or plan to become pregnant.
- are breastfeeding or wish to breastfeed.
- drink alcohol regularly. Alcohol may increase the effects of ALEPAM.
- plan to have surgery.
Suddenly stopping ALEPAM in patients with epilepsy can cause a temporary increase in the number and severity of seizures.
Your doctor may have prescribed ALEPAM for depression or psychosis. ALEPAM is not recommended as the first choice of treatment for depression and psychosis. It may increase depression, worsen mental illness, suicidal thoughts and actions.
Your doctor may want to take special care if you have any of these conditions.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
ALEPAM should not be used during pregnancy.
It should be avoided during the first trimester of pregnancy as it may increase the risk of defects present at birth.
ALEPAM may cause unwanted effects in the newborn baby if taken during the late phase of pregnancy or during childbirth, such as low muscle strength, shallow breathing and feeding problems.
Your doctor will discuss the risks and benefits of taking ALEPAM during pregnancy.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
ALEPAM passes into breast milk and may cause drowsiness and/or feeding difficulties in the baby. Your doctor will discuss the risks and benefits of taking ALEPAM when breastfeeding.
Children under 16 years of age
ALEPAM is not recommended for use in children under 16 years of age, as its safety and effectiveness have not been established in this age group.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with ALEPAM and affect how it works.
- other sleeping tablets, sedatives or tranquillisers
- medicines for depression, schizophrenia and other mental illnesses
- medicines to treat epilepsy and fits
- antihistamines, medicines for allergies, colds or travel sickness
- some medicines used to treat Parkinson's disease
- muscle relaxants
- strong pain relievers.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect ALEPAM.
4. How do I use ALEPAM?
How much to take
- The dose varies from person to person.
- Your doctor will tell you how many tablets you need to take each day and when to take them.
- This depends on your condition and whether or not you are taking any other medicines.
When to take ALEPAM
Take ALEPAM only for as long as your doctor recommends.
Usually, ALEPAM should be taken for short periods only (for example 2 to 4 weeks). Continuous long-term use is not recommended unless advised by your doctor. The use of benzodiazepines may lead to dependence on the medicine.
How to take ALEPAM
- Swallow the tablets with a glass of water.
- ALEPAM can be taken with or without food.
If you forget to take ALEPAM
ALEPAM should be used regularly at the same time each day.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much ALEPAM
If you think that you have used too much ALEPAM, you may need urgent medical attention.
If you take too much ALEPAM, you may feel drowsy, tired, confused, dizzy, have low muscle strength, low blood pressure, have difficulty breathing, lack of coordination, feel weak or become unconscious. It can be rarely fatal.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while taking ALEPAM?
Things you should do
- Take ALEPAM exactly as your doctor has prescribed.
- Visit your doctor regularly so they can check on your progress. Your doctor will check your condition to see whether you should continue to take it.
- If you have to have any blood tests, tell your doctor that you are taking ALEPAM. ALEPAM may affect the results of some tests.
- Keep enough of your medicine to last weekends and holidays.
Be careful if you are elderly, unwell or taking other medicines.
You may have an increased chance of getting side effects such as drowsiness, confusion, dizziness and unsteadiness, which may increase the risk of a fall.
Call your doctor straight away if you:
- become pregnant while taking ALEPAM, tell your doctor immediately.
- feel ALEPAM is not helping your condition or if you have any problems.
All thoughts of suicide must be taken seriously. Tell your doctor or a mental health professional immediately if you have any suicidal thoughts or other mental/mood changes.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking ALEPAM.
Things you should not do
- Do not take ALEPAM for a longer time than your doctor has prescribed.
- Do not use ALEPAM to treat any other conditions unless your doctor tells you to.
- Do not give ALEPAM to anyone else, even if they have the same condition as you.
Do not stop taking ALEPAM, or change the dose, without checking with your doctor.
Stopping ALEPAM suddenly may cause some unwanted effects. It is more common in patients that have received high doses over longer periods of time.
Withdrawal symptoms include insomnia, anxiety, unusual mood, panic attacks, dizziness, light sensitivity, confusion, seeing or hearing things that are not real (hallucinations), vomiting, sweating, fits (convulsions), a feeling of loss of identity/feeling detached from yourself (depersonalisation or derealisation) and loss of short-term memory.
Your doctor may want you to gradually reduce the amount of ALEPAM you are taking before stopping completely. This may help reduce the possibility of unwanted side effects.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how ALEPAM affects you.
ALEPAM may cause drowsiness or dizziness in some people. If either of these occurs, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol regularly.
Combining ALEPAM and alcohol can make you more drowsy or dizzy. Your doctor may suggest that you avoid alcohol while you are taking ALEPAM.
Looking after your medicine
- Keep your tablets in their blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
- Keep your tablets in a cool dry place where the temperature stays below 30°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If your doctor tells you to stop taking ALEPAM, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What ALEPAM contains
| Active ingredient (main ingredient) | oxazepam |
| Other ingredients (inactive ingredients) | lactose monohydrate maize starch quinoline yellow aluminium lake erythrosine aluminium lake magnesium stearate. |
| Potential allergens | sugars as lactose and trace amounts of sulfites |
Do not take this medicine if you are allergic to any of these ingredients.
What ALEPAM looks like
ALEPAM 15 - 8 mm pale yellow flat bevelled edged tablet marked OM/15 on one side, G on reverse. Each bottle contains 25 tablets Each blister pack carton contains 25 tablets with hospital only packs containing 90 tablets (AUST R 17572).
ALEPAM 30 - 8 mm pale orange flat bevelled edged tablet marked OM/30 on one side, G on reverse. Each bottle contains 25 tablets. Each blister pack carton contains 25 tablets. (AUST R 385082).
Who distributes ALEPAM
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in February 2024.
ALEPAM_cmi\Feb24/00
Published by MIMS March 2024


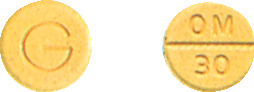
 Chemical name: (3RS)-7-chloro-3- hydroxy-5-phenyl-1,3-dihydro-2H- 1,4-benzodiazepin-2-one.
Chemical name: (3RS)-7-chloro-3- hydroxy-5-phenyl-1,3-dihydro-2H- 1,4-benzodiazepin-2-one.