What is in this leaflet
This leaflet answers some common questions about ALKERAN tablets. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking ALKERAN against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ALKERAN is used for
ALKERAN contains melphalan as the active ingredient.
ALKERAN belongs to a group of medicines called cytotoxics and is used to treat some types of cancer and certain blood disorders.
It interferes with the growth of cancer cells.
ALKERAN may be used in combination with other medicines to treat cancer.
Ask your doctor if you have any questions about why ALKERAN has been prescribed for you.
Your doctor may have prescribed it for another purpose.
This medicine is only available with a doctor's prescription.
There is no evidence that it is addictive.
Before you take it
When you must not take it
Do not take ALKERAN if you have ever had an allergic reaction to:
- melphalan
- any of the tablet ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty in breathing; swelling of the face, lips, tongue or any other parts of the body; rash, itching or hives on the skin.
Do not take it if you have an infection or a high temperature.
Your doctor may decide to delay your treatment until the infection has gone. A mild illness, such as a cold, is not usually a reason to delay treatment.
Do not take ALKERAN if you are planning to become pregnant or father a child.
ALKERAN may harm eggs and sperm. Reliable contraceptive methods must be taken to avoid pregnancy whilst you or your partner is taking this medicine and for at least 12 weeks after either partner stops using it.
Do not take ALKERAN if you are pregnant or breast feeding unless you and your doctor have discussed the risks and benefits involved.
Do not take ALKERAN after the expiry date (EXP) printed on the bottle label.
Do not take it if the bottle shows signs of tampering.
Before you start to take it
Tell your doctor if you are allergic to any other medicines or any other foods, dyes or preservatives.
Tell your doctor if you have or have had any of the following conditions:
- you have recently received or are receiving radiotherapy or chemotherapy
- you have recently been vaccinated or are planning to be vaccinated
- kidney disease
- blood disorders
- you have received ALKERAN treatment previously.
If you have not told your doctor about any of the above, tell them before you start taking ALKERAN.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your supermarket, pharmacy or health food shop.
Some medicines may be affected by ALKERAN or may affect how well it works. You may need to take different amounts of your medicine or you may need to take different medicines. These include:
- nalidixic acid
- cyclosporin
- cisplatin
- carmustine
- other cytotoxic drugs
- vaccinations with 'live' organism vaccines.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking this medicine.
How to take it
How much to take
Take ALKERAN tablets exactly as directed by your doctor.
Your doctor will decide what dose and for how long you will be taking ALKERAN. This depends on factors such as your weight, any pre-existing conditions and your response to the treatment. Your doctor may change the dose and frequency of your medicine as your condition changes.
Your doctor may order regular blood tests while you are taking ALKERAN in order to monitor your blood cell count and to change your dosage if necessary.
How to take it
Swallow each tablet whole with water.
Do not break, crush or chew the tablets.
How long to take it for
Do not stop taking ALKERAN tablets, or change the dose without first checking with your doctor.
It is important to take your ALKERAN tablets until your doctor tells you to stop.
Use in Children
The safety and effectiveness in children has not been established.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much ALKERAN. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are taking it
Things you must do
Tell your doctor if, for any reason, you have not taken your medicine exactly as directed.
Otherwise your doctor may think that it was not working as it should and change your treatment unnecessarily.
Do not take a double dose to make up for the one you have missed.
It is important that you visit your doctor regularly so your doctor can check your progress and make sure your medicine is working.
Tell any other specialist, doctor, dentist or pharmacist that you are on ALKERAN, especially if you are about to be started on any new medicines, immunisations, vaccinations or radiotherapy.
Tell your doctor if you become pregnant, are trying to become pregnant, or trying to father a child.
If you are about to undergo surgery or an operation, tell your doctor or surgeon that you are taking ALKERAN.
Things you must not do
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Do not use ALKERAN tablets to treat any other complaints unless your doctor says to.
Things to be careful of
Do not break, crush or chew the tablet.
Provided the outer coating of the tablet is intact, there is no risk in handling ALKERAN tablets.
Be careful driving or operating machinery until you know how ALKERAN tablets affect you.
Do not have any vaccinations without your doctor's approval.
ALKERAN may lower your body's resistance and there is a chance you might get the infection the vaccination is meant to prevent.
ALKERAN tablets can lower the number of white blood cells and platelets in your blood. This means that you have an increased chance of getting an infection or bleeding. The following precautions should be taken to reduce your risk of infection or bleeding:
- avoid people who have infections
Check with your doctor immediately if you think you may be getting an infection, or if you get a fever, chills, cough, hoarse throat, lower back or side pain or find it painful or difficult to urinate.
- be careful when using a toothbrush, tooth pick or dental floss
Check with your doctor before having dental work.
Your doctor, dentist, nurse or pharmacist may recommend other ways to clean your teeth and gums.
- be careful not to cut yourself when using sharp objects such as a razor or nail cutters
- avoid contact sports or other situations where you may bruise or get injured.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking ALKERAN.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
The following side-effects have been reported with ALKERAN:
- production of bone marrow cells may be reduced. You may notice an increase in infections. Your doctor will do regular blood tests, but you should tell him at once if you notice any signs of fever or infection or any unexpected bruising, bleeding or signs of blood in your urine.
- in women, periods may stop
- in men, sperm production may be reduced or stopped.
Tell your doctor if you notice any of the following:
- nausea and vomiting
- diarrhoea
- skin rash or itching
- frequent infections such as fever, sore throat or mouth ulcers
- bruising or bleeding more easily than normal, nose bleeds
- unusual tiredness, looking pale, feeling weaker, dizzy or more tired than usual
- jaundice, a yellowing of the whites of the eyes or skin
- persistent cough or breathlessness
- hair loss
- unusually fast heart beat.
Tell your doctor immediately if you notice any of the following:
- wheezing, swelling of the lips or mouth, difficulty in breathing, hayfever, lumpy rash (hives) or fainting.
These could be a symptom of an allergic reaction.
Tell your doctor if you notice anything else that is making you feel unwell, even if you think the problems are not connected with this medicine and are not referred to in this leaflet.
Other side effects not listed above may also occur in some people.
Do not be alarmed by this list of side effects.
You may not experience any of them.
After taking it
Storage
Store ALKERAN tablets in the refrigerator where the temperature is between 2 and 8 degrees Celsius. Do not freeze.
Keep it where young children cannot reach it.
Keep your tablets in the bottle until it is time to take them. If you take the medicine out of the bottle it may not keep as well.
Product description
What it looks like
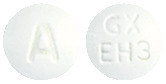
ALKERAN tablets are white to off-white, film-coated, round tablets engraved with "GX EH3" on one side and "A" on the other.
Available in bottles of 25 tablets.
Ingredients
Active ingredient:
Each ALKERAN tablet contains 2 mg melphalan.
Inactive ingredients:
- cellulose-microcrystalline
- crospovidone
- silica colloidal anhydrous
- magnesium stearate
- hypromellose
- titanium dioxide
- macrogol 400.
ALKERAN tablets do not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Aspen Pharmacare Australia Pty Limited
34-36 Chandos Street
St Leonards NSW 2065 Australia
Australian registration number: AUST R 81543
This leaflet was prepared in August 2010.
Published by MIMS November 2010


 One hundred and seven patients were randomised to the oral melphalan arm and 203 patients to the IV melphalan arm. More patients had a poor-risk classification (58% versus 44%) and high tumour load (51% versus 34%) on the oral compared to the IV arm (P < 0.04). Response rates at week 22 are shown in Table 3.
One hundred and seven patients were randomised to the oral melphalan arm and 203 patients to the IV melphalan arm. More patients had a poor-risk classification (58% versus 44%) and high tumour load (51% versus 34%) on the oral compared to the IV arm (P < 0.04). Response rates at week 22 are shown in Table 3. Because of changes in protocol design after week 22, other efficacy parameters such as response duration and survival cannot be compared.
Because of changes in protocol design after week 22, other efficacy parameters such as response duration and survival cannot be compared.
