SUMMARY CMI
ANAMORPH® tablets
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
WARNING: Important safety information is provided in a boxed warning in the full CMI. Read before using this medicine.
1. Why am I using ANAMORPH?
ANAMORPH contains the active ingredient morphine sulfate pentahydrate. ANAMORPH is used for the treatment of chronic severe pain of cancer.
For more information, see Section 1. Why am I using ANAMORPH? in the full CMI.
2. What should I know before I use ANAMORPH?
Do not use if you have ever had an allergic reaction to morphine or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use ANAMORPH? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with ANAMORPH and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use ANAMORPH?
- Your doctor will tell you exactly how much to take.
- Follow the instructions given to you by your doctor or your pharmacist.
- You must only take ANAMORPH by mouth.
More instructions can be found in Section 4. How do I use ANAMORPH? in the full CMI.
5. What should I know while using ANAMORPH?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using ANAMORPH? in the full CMI.
6. Are there any side effects?
ANAMORPH may cause constipation, nausea, vomiting, dizziness, drowsiness and be habit forming if taken frequently or over long periods.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
WARNING:
Limitations of use
ANAMORPH should only be used when your doctor decides that other treatment options are not able to effectively manage your pain or you cannot tolerate them.
Hazardous and harmful use
ANAMORPH poses risks of abuse, misuse and addiction which can lead to overdose and death. Your doctor will monitor you regularly during treatment.
Life threatening respiratory depression
ANAMORPH can cause life-threatening or fatal breathing problems (slow, shallow, unusual or no breathing) even when used as recommended. These problems can occur at any time during use, but the risk is higher when first starting ANAMORPH and after a dose increase, if you are older, or have an existing problem with your lungs. Your doctor will monitor you and change the dose as appropriate.
Use of other medicines while using ANAMORPH
Using ANAMORPH with other medicines that can make you feel drowsy such as sleeping tablets (e.g. benzodiazepines), other pain relievers, antihistamines, antidepressants, antipsychotics, gabapentinoids (e.g. gabapentin and pregabalin), cannabis and alcohol may result in severe drowsiness, decreased awareness, breathing problems, coma and death. Your doctor will minimise the dose and duration of use; and monitor you for signs and symptoms of breathing difficulties and sedation. You must not drink alcohol while using ANAMORPH.
FULL CMI
ANAMORPH® tablets
Active ingredient: morphine sulfate pentahydrate
Consumer Medicine Information (CMI)
This leaflet provides important information about using ANAMORPH. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using ANAMORPH.
Where to find information in this leaflet:
1. Why am I using ANAMORPH?
2. What should I know before I use ANAMORPH?
3. What if I am taking other medicines?
4. How do I use ANAMORPH?
5. What should I know while using ANAMORPH?
6. Are there any side effects?
7. Product details
1. Why am I using ANAMORPH?
ANAMORPH contains the active ingredient morphine sulfate pentahydrate. Morphine belongs to a group of medicines called opioid analgesics.
ANAMORPH is used for the treatment of chronic severe pain of cancer.
2. What should I know before I use ANAMORPH?
Warnings
Do not use ANAMORPH if:
- you are allergic to morphine, or any of the ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can use this medicine.
- you have acute breathing difficulties such as bronchitis or asthma
- you have severe abdominal pain with bloating, cramps or vomiting
- you have a condition where your small bowel does not work properly
- you take medicine for depression called a 'monoamine oxidase inhibitor' or have taken any in the last two weeks
- you are pregnant or in labour.
Check with your doctor if you:
- are severely drowsy, have a reduced level of consciousness or are feeling faint or dizzy upon standing
- have heart problems or heart disease
- have low blood pressure
- have chronic lung disease
- suffer from sleep apnoea (temporarily stop breathing while you sleep)
- have just drunk a large amount of alcohol, regularly drink large amounts of alcohol or have confusion and shaking due to stopping drinking alcohol
- suffer from convulsions, fits or seizures
- have a head injury, brain tumour or increased pressure in your head
- are about to have surgery, had recent gastrointestinal surgery or have had other surgery in the last 24 hours
- have chronic liver or kidney disease
- have increased prostate size or difficulty passing urine
- have problems with your gall bladder
- have problems with or recent surgery of your bile duct
- have inflammation of the pancreas
- have adrenal glands which are not working properly
- have an underactive thyroid gland
- have a severe mental condition involving losing contact with reality or an inability to think clearly
- have an addiction or history of abuse of alcohol, opioids or other drugs.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
ANAMORPH given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
Talk to your doctor if you are breastfeeding or intend to breastfeed. Low levels of opioid analgesics have been detected in human milk.
Addiction
You can become addicted to ANAMORPH even if you take it exactly as prescribed. ANAMORPH may become habit forming causing mental and physical dependence. If abused, it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking ANAMORPH. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking ANAMORPH suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to ANAMORPH may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with ANAMORPH and affect how it works.
Using ANAMORPH with medicines that can make you feel drowsy may result in severe drowsiness, decreased awareness, breathing problems, coma and death. These medicines include:
- sleeping tablets and other sedatives (including benzodiazepines and barbiturates)
- gabapentinoids
- cannabis
- antihistamines
- anxiolytics
- general anaesthetics
- antiemetics
- antidepressants (including tricyclic antidepressants)
- antipsychotics (including phenothiazines)
- neuroleptics
- beta-blockers (medicines used to treat high blood pressure)
- other opioids
- alcohol.
ANAMORPH may enhance the action of neuromuscular blocking agents (medicines used to relax muscles) and affect your breathing.
ANAMORPH may increase the anticoagulant activity of coumarin and other anticoagulants (medicines used to prevent blood clots).
ANAMORPH should not be used if you are taking non-selective monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping such treatment.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect ANAMORPH.
4. How do I use ANAMORPH?
How much to take
- Your doctor will tell you how much to take.
- Follow the instructions provided and use ANAMORPH until your doctor tells you to stop.
When to take ANAMORPH
- Take ANAMORPH every 4-6 hours or as directed by your doctor.
- Take ANAMORPH at about the same time each day.
How to take ANAMORPH
- Swallow ANAMORPH tablets with a glass of water.
- ANAMORPH can be taken before or after food, but try to take it the same way every time.
If you begin to experience pain, tell your doctor as your dosage may have to be reviewed.
If you forget to use ANAMORPH
If you are taking regular doses of ANAMORPH, you should take it at the same time each day. If you miss your dose at the usual time, you may take ANAMORPH as soon as you remember or think you need it.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of getting unwanted side effects including severe drowsiness, decreased awareness, breathing problems, coma and death.
If you use too much ANAMORPH (overdose)
If you or someone else receive too much (overdose), and experience one or more of the symptoms below, call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally used ANAMORPH that was prescribed for you. If someone takes an overdose, they may experience one or more of the following symptoms:
- slow, unusual or difficult breathing
- drowsiness, dizziness or unconsciousness
- slow or weak heartbeat
- nausea or vomiting
- convulsions or fits.
If you think you or someone else may have used too much ANAMORPH you should immediately:
- phone the Poisons Information Centre (by calling 13 11 26), or
- contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this leaflet and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
5. What should I know while using ANAMORPH?
Things you should do
Call your doctor straight away if you:
- become pregnant
- feel your pain is getting worse.
Remind any doctor or dentist or pharmacist you visit that you are using ANAMORPH.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
Things you should not do
Do not stop using this medicine suddenly. If you stop taking ANAMORPH suddenly, your pain may worsen and you may experience withdrawal symptoms.
Do not take ANAMORPH to treat any other complaint unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how ANAMORPH affects you.
ANAMORPH may cause drowsiness or impair mental and/or physical ability in some people.
Drinking alcohol
Tell your doctor if you drink alcohol.
Alcohol may make you feel more sleepy, and could increase the risk of serious side effects, such as shallow breathing with the risk of stopping breathing and loss of consciousness.
Looking after your medicine
- Store below 30°C.
Follow the instructions on the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Gastrointestinal and urinary related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Breathing related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What ANAMORPH contains
| Active ingredient (main ingredient) | Morphine sulfate pentahydrate |
| Other ingredients (inactive ingredients) | Lactose Croscarmellose sodium Magnesium stearate |
| Potential allergens | Sugars as Lactose |
Do not take this medicine if you are allergic to any of these ingredients.
What ANAMORPH looks like
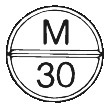
ANAMORPH 30 mg tablets are round, white, flat bevelled edge tablets, scored on one side and marked with “M” above the breakline and “30” below the breakline; (Aust R 34032)
Who distributes Anamorph
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
This leaflet was prepared in October 2023
Published by MIMS November 2023