What is in this leaflet
This leaflet answers some common questions about clarithromycin. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What this medicine is used for
Clarithromycin belongs to a group of medicines called macrolide antibiotics.
Clarithromycin is used to treat certain bacterial infections, such as:
- respiratory tract infections
- skin infections
- peptic ulcers
Clarithromycin is also used to prevent a specific bacterial infection associated with HIV infection.
How it works
Clarithromycin works by killing or stopping the growth of bacteria which causes infections.
This medicine will not work against infection caused by viruses, such as cold or flu.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
This medicine is not addictive.
These tablets are not recommended for children under 12 years of age.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- clarithromycin
- other macrolide antibiotics (e.g. erythromycin, roxithromycin, azithromycin)
- any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you have or have had any of the following medical conditions:
- irregular heartbeat, including Torsades de pointes
- severe liver and kidney problems
- liver or kidney problems, and taking a p-glycoprotein inhibitor or a strong CYP3A4 inhibitor
- hypokalaemia (low potassium).
Do not take this medicine if you are taking any of the following medicines:
- astemizole or terfenadine, used to treat allergy symptoms
- cisapride, used to treat stomach problems
- pimozide, used to treat psychotic disorders
- ergotamine or dihydroergotamine, used to treat migraines
- lovastatin or simvastatin, used to treat high cholesterol
- midazolam
- ticagrelor or ranolazine
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- liver, kidney or heart problems
- low potassium or magnesium
- myasthenia gravis
- pneumonia
- skin and soft tissue infections.
Tell your doctor if you are pregnant, plan to become pregnant or are breastfeeding. Do not take this medicine until you and your doctor have discussed the risks and benefits involved.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interact with clarithromycin. These include:
- medicines used to prevent blood clotting (e.g. warfarin, cilostazol)
- some medicines used for epilepsy (e.g. phenytoin, valproate, carbamazepine, hexobarbital)
- theophylline, used to treat asthma
- some medicines used to treat heart complaints (e.g. digoxin, quinidine, disopyramide, verapamil, amlodipine, diltiazem)
- medicines used to treat sleeplessness such as triazolam
- alprazolam, used to treat anxiety
- anti-viral medicines (e.g. zidovudine, ritonavir, indinavir, atazanavir, saquinavir, efavirenz, nevirapine, etravirine)
- some antibiotics (e.g. rifampicin, rifabutin, aminoglycosides)
- itraconazole and ketoconazole, used to treat fungal infections
- fluoxetine, used for treating depression
- colchicine, used to treat gout
- some medicines used to treat erection problems (e.g. sildenafil, tadalafil, vardenafil)
- vinblastine, used to treat certain cancers
- some medicines used to suppress the immune system (e.g. cyclosporin, tacrolimus, methylprednisolone)
- atorvastatin and rosuvastatin, used for lowering cholesterol
- omeprazole, used in treating stomach ulcers
- tolterodine, used to treat urinary incontinence
- medicines used to treat diabetes, (e.g. insulin, repaglinide, nateglinide, pioglitazone, rosiglitazone)
- herbal medicines (e.g. St John's Wort)
If you are taking any of these, you may need a different dose, or you may need to take different medicines.
Other medicines not listed above may also interact with clarithromycin.
How to take this medicine
Follow all directions given to you by your doctor and pharmacist carefully. They may differ to the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much of this medicine you should take, depending on your condition and if you are taking any other medicines.
For respiratory tract infections and skin infections, the usual adult dose is one clarithromycin 250 mg tablet twice a day.
For more severe infections, the dose may be increased.
For respiratory tract infections, the usual dose for children is 7.5mg/kg twice a day.
Your doctor may adjust the amount or frequency of the dose according to the infection being treated and the severity of your condition.
How to take it
Swallow the tablet whole with a full glass of water.
When to take it
Take your medicine at about the same time each day. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take it with or without food.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
If you are being treated for an infection, clarithromycin is usually taken for one or two weeks.
Do not stop taking clarithromycin, even if you feel better after a few days, unless advised by your doctor. Your infection may not clear completely if you stop taking your medicine too soon.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you experiencing side effects.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to the Emergency department at your nearest hospital if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much clarithromycin, you may develop severe stomach problems, liver problems or experience an allergic reaction.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are taking this medicine for an infection and your symptoms do not improve within a few days, or if they become worse, tell your doctor.
Tell your doctor immediately if you get severe diarrhoea. Do this even if it occurs several weeks after stopping clarithromycin. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take any medicine to stop your diarrhoea without first checking with your doctor.
If you get a sore white mouth or tongue while taking or soon after stopping clarithromycin, tell your doctor. Also tell your doctor if you get vaginal itching or discharge. This may mean you have a fungal infection called thrush. Sometimes the use of this medicine allows fungi to grow and the above symptoms to occur.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects.
Things you must not do
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not take your medicine to treat any other complaint unless your doctor tells you to.
Do not stop taking your medicine, or change the dosage, without first checking with your doctor. Your infection may not clear completely if you stop taking your medicine too soon.
Things to be careful of
Be careful driving or operating machinery until you know how clarithromycin affects you.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking clarithromycin.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following:
- stomach cramps and pains
- nausea, vomiting and severe diarrhoea
- oral thrush or vaginal thrush
- change in taste sensation
- headache
Tell your doctor as soon as possible if you notice any of the following:
- yellowing of the eyes or skin (jaundice)
- feeling generally unwell and having poor appetite
- hearing disturbances
- chest pain
- dizziness, confusion, hallucinations, convulsions
- any type of skin rash, itching, hives
- severe diarrhoea, especially if bloody
- severe upper stomach pain with nausea and vomiting (pancreatitis)
- unexpected muscle pain, tenderness or weakness not caused by exercise.
These may be serious side effects and you may need medical attention.
Tell your doctor immediately if you notice any of the following side effects, even if they occur several weeks after stopping treatment with clarithromycin:
- severe stomach or abdominal cramps
- watery and severe diarrhoea, which may also be bloody
- fever, in combination with one or both of the above.
These are serious side effects. You may have a serious condition affecting your bowel and you may need urgent medical care.
Do not take any diarrhoea medicine without first checking with your doctor.
Other side effects not listed above may occur in some patients.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Allergic reactions
If you think you are having an allergic reaction to clarithromycin, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
Storage and disposal
Storage
Keep your medicine in its pack until it is time to take it. If you take the tablets out of their pack, they may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 30°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine left over.
Product description
What it looks like
250 mg tablets:
Pale yellow, oval, film-coated tablets engraved "CLA250" on one side, "APO" on the other side.
Available in blister packs of 2, 14, 28 and 100 tablets. AUST R 134850
Available in bottles of 14, 28 and 100 tablets. AUST R 134865
500 mg tablets:
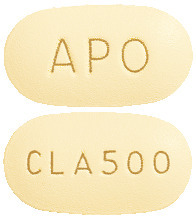
Pale yellow, capsule-shaped, film-coated tablets engraved "CLA500" on one side, "APO" on the other side.
Available in blister packs of 3, 14, 20, 28, 42 and 100 tablets. AUST R 134856
Available in bottles of 14, 20, 28, 42 and 100 tablets. AUST R 134883
* Not all strengths, pack sizes and/or pack types may be available.
Ingredients
Each tablet contains 250 mg or 500 mg of clarithromycin as the active ingredient.
It also contains the following:
- microcrystalline cellulose
- croscarmellose sodium
- magnesium stearate
- colloidal anhydrous silica
- hypromellose
- iron oxide yellow E172
- titanium dioxide E171
- macrogol 8000
This medicine does not contain gluten, lactose, sucrose, tartrazine or any other azo dyes.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
APO and APOTEX are registered trademarks of Apotex Inc.
This leaflet was prepared in March 2019.
Published by MIMS May 2019





 Information was obtained regarding the penetration of clarithromycin in middle ear fluid in paediatric patients with otitis media. Approximately 2.5 hours after receiving the fifth dose (dosage was 7.5 mg/kg twice a day) the mean concentration of clarithromycin was 2.53 microgram/g fluid in the middle ear, and for the 14-hydroxy metabolite was 1.27 microgram/g. The concentrations of parent drug and 14-OH metabolite were variable, with two-thirds of patients having levels greater than the corresponding concentration in serum and one-third of patients having levels similar or lower. The mean ratio was 2.48 ± 3.57.
Information was obtained regarding the penetration of clarithromycin in middle ear fluid in paediatric patients with otitis media. Approximately 2.5 hours after receiving the fifth dose (dosage was 7.5 mg/kg twice a day) the mean concentration of clarithromycin was 2.53 microgram/g fluid in the middle ear, and for the 14-hydroxy metabolite was 1.27 microgram/g. The concentrations of parent drug and 14-OH metabolite were variable, with two-thirds of patients having levels greater than the corresponding concentration in serum and one-third of patients having levels similar or lower. The mean ratio was 2.48 ± 3.57.
 There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions, Colchicine; Section 4.4 Special Warnings and Precautions for Use).
There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions, Colchicine; Section 4.4 Special Warnings and Precautions for Use).