What is in this leaflet
This leaflet answers some common questions about fluconazole. It does not contain all the available information. It does not take the place of talking to your pharmacist.
All medicines have risks and benefits. Your pharmacist has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What this medicine is used for
Fluconazole is used to treat a fungal infection known as vaginal thrush (vaginal candidiasis). Fluconazole belongs to a group of medicines known as azole antifungals.
Vaginal thrush is an infection caused by a yeast-like fungus called Candida. Candida is one of the many organisms that live in the vagina and its growth is normally balanced by your body's immune system. If this natural balancing is upset, Candida can multiply in the vagina to cause the symptoms of thrush.
How it works
Fluconazole works by preventing the growth of the fungi causing your infection.
Ask your pharmacist if you have any questions about why this medicine has been prescribed for you. Your pharmacist may have prescribed this medicine for another reason
This medicine is available without a doctor's prescription, but your pharmacist's advice is required.
This medicine is not addictive.
This medicine is not recommended for children under 18 years of age, except under doctor advice.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- fluconazole
- any other azole antifungals e.g. clotrimazole
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you are taking any of the following medicines:
- terfenadine (only in patients receiving fluconazole at multiple doses of 400mg/day)
- cisapride
- astemizole
- erythromycin
- quinidine
- pimozide
- voriconazole
Do not take this medicine if you are pregnant or plan to become pregnant. Fluconazole may affect your developing baby if you take it during pregnancy. Your pharmacist will discuss with you the risks and benefits involved.
Do not use this medicine if you are a female of childbearing age unless you are using adequate contraception. Effective contraception should be taken during treatment and for about 1 week after the final dose.
Do not take this medicine if you are breast-feeding. Fluconazole may pass into breast milk and affect your baby.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your pharmacist.
Before you start to take it
Tell your pharmacist if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your pharmacist if you have or have had any of the following medical conditions:
- abnormal or irregular vaginal bleeding or blood-stained discharge
- vulvar or vaginal sores, ulcers or blisters
- lower abdominal pain or burning when passing urine
- liver, kidney or heart problems
- low potassium levels in the blood
- adrenal problems
- lactose, or galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption. This product contains lactose.
Tell your pharmacist if you are planning to have an operation. Some medicines used during anaesthetics may interact with fluconazole.
If you have not told your pharmacist about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interact with fluconazole. These include:
Medicines not to be taken with fluconazole:
- astemizole, used for allergies
- cisapride, used to treat stomach problems
- quinidine, used to treat heart problems
- erythromycin, an antibiotic
- pimozide, used to treat mood disorders
- voriconazole, an antifungal medicine
Medicines to be taken with care with fluconazole:
- terfenadine (do not take this medicine if you are receiving fluconazole at multiple doses of 400mg/day)
- amiodarone, used for heart problems
- some medicines used to treat diabetes (e.g. glipizide, chlorpropamide, tolbutamide, glibenclamide, glimepiride, gliclazide, pioglitazone, rosiglitazone)
- some antibiotics and antiviral drugs (e.g. erythromycin, amphotericin B, rifampicin. rifabutin, zidovudine, saquinavir)
- some medicines used to suppress the immune system (e.g. ciclosporine, tacrolimus, sirolimus, prednisone)
- some medicines used to treat cancer (e.g. cyclophosphamide, ibrutinib, olaparib, vincristine, vinblastine)
- vitamin A
- antidepressants (e.g. amitriptyline, nortriptyline)
- warfarin or ticlopidine, used to stop blood clots
- phenytoin and carbamazepine used to treat epilepsy
- theophylline, used to treat asthma
- medicines used during anaesthetics (e.g. alfentanyl, midazolam, fentanyl)
- benzodiazepines (e.g. triazolam)
- hydrochlorothiazide, used for treating fluid problems
- medicines used to treat high blood pressure (e.g. losartan, amlodipine, felodipine)
- medicines used to treat high cholesterol (e.g. simvastatin, fluvastatin, atorvastatin)
- medicines to treat low levels of sodium in the blood (e.g. tolvaptan)
- some medicines used for pain relief (e.g. methadone, celecoxib)
- halofantrine, used to treat malaria
- the contraceptive pill
Talk to your doctor or pharmacist about the need for an additional method of contraception while taking fluconazole.
If you are taking any of these, you may need a different dose, or you may need to take different medicines.
Other medicines not listed above may also interact with fluconazole.
How to take this medicine
Follow all directions given to you by your pharmacist carefully. They may differ to the information contained in this leaflet.
How much to take
A single dose of one capsule.
How to take it
Swallow the whole capsule with a glass of water.
When to take it
It does not matter whether you take this medicine with or without food.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency Department at your nearest hospital. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you have taken this medicine.
Tell any other doctors, dentists and pharmacists who are treating you that you have taken this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If the symptoms of your infection do not improve after 3 days, or if they become worse, tell your doctor or pharmacist.
Things you must not do
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not take your medicine to treat any other complaint unless your doctor or pharmacist tells you to.
Things to be careful of
Tell your doctor or pharmacist immediately if you develop a rash soon after taking this medicine. People with AIDS or weak immune system may be more prone to serious side effects of the skin.
What you can do to avoid thrush in the future
- avoid wearing synthetic underwear
- wear loose-fitting cotton briefs, stockings
- wash the area regularly, but do not wash and dry yourself harshly
- avoid vaginal deodorants, perfumed soaps and bath additives
Ask your doctor or pharmacist for more information for things you can do to avoid thrush in the future.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking fluconazole.
This medicine helps most people with vaginal thrush, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- nausea or vomiting
- stomach pain, indigestion, wind
- diarrhoea
- muscle or back pain
- headache
The above list includes the more common side effects. Mostly, these are mild.
Tell your doctor or pharmacist as soon as possible if you notice any of the following:
- skin reactions or rash
- unusual muscle stiffness causing poor control of movement
- frequent infections, such as fever, severe chills, sore throat or mouth ulcers
- bleeding or bruising more easily than normal
- passing more urine than normal, kidney pain (pain on the sides of the body)
- yellowing of the skin or eyes (jaundice); dark urine, pale stools; loss of appetite; unusual tiredness (signs of liver disease)
The above list includes serious side effects. You may need medical attention. Most of these side effects are rare.
If you experience any of the following, contact your doctor immediately or go to Accident and Emergency at your nearest hospital:
- shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin (signs of an allergic reaction)
- fast, slow or irregular heart beat or palpitations and/or fainting.
- severe blisters and bleeding of the lips, eyes, mouth, nose and genitals
- a severe rash with skin peeling, fever, chills and aching muscles.
The above list includes very serious side effects that are usually very rare. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may occur in some patients.
Storage and disposal
Storage
Keep your medicine in its pack until it is time to take it. If you take your medicine out of its pack it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If the medicine has passed its expiry date, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
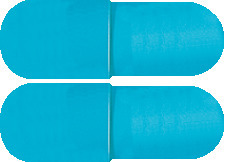
Hard gelatine capsule of size '1' with sky blue body and cap.
Each pack contains 1 capsule.
AUST R 152959.
Ingredients
Each capsule contains 150 mg of fluconazole as the active ingredient.
It also contains the following:
- lactose monohydrate
- maize starch
- colloidal anhydrous silica
- purified talc
- sodium lauryl sulphate
- gelatin
- titanium dioxide
- patent blue V
This medicine does not contain gluten, sucrose, tartrazine or any other azo dyes.
This medicine contains sugars (as lactose) and sulfites.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
Australia
Tel: +61 2 8877 8333
Web: www1.apotex.com/au
The APOTEX and APOHEALTH trademarks are used under licence.
This leaflet was updated in December 2020.
Published by MIMS March 2021




 Molecular Formula: C13H12F2N6O.
Molecular Formula: C13H12F2N6O.