What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some of the common questions about lercanidipine. It does not contain all the available information. It does not replace seeking advice from your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking lercanidipine against the benefits this medicine is expected to have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is APO-Lercanidipine. It contains the active ingredient, lercanidipine (as lercanidipine hydrochloride).
Lercanidipine belongs to a group of medicines called dihydropyridine calcium channel blockers.
Lercanidipine helps lower high blood pressure, otherwise known as hypertension.
This medicine is available only with a doctor's prescription.
How it works
This medicine works by relaxing some of the blood vessels in the body and reducing resistance to the flow of blood through the blood vessels.
Everyone has blood pressure. This pressure helps get your blood all around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. If your blood pressure stays higher than is needed, even when you are calm and relaxed, you have hypertension (high blood pressure).
There are usually no symptoms of hypertension. The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. If high blood pressure is not treated it can lead to serious health problems. You may feel fine and have no symptoms, but hypertension can cause stroke, heart disease and kidney failure.
There is no evidence that this medicine is addictive.
Use in children
This medicine should not be used in children.
Before you take this medicine
When you must not take it
Do not take this medicine if:
You have or have had any of the following:
- severe liver or kidney disease.
- taking another medicine called cyclosporine.
- You are hypersensitive to, or have had an allergic reaction to, lercanidipine or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include: cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin; fainting; or hay fever-like symptoms.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- liver or kidney disease or you are on dialysis
- other heart conditions such as: heart disease, uncontrolled heart failure, an obstruction to the flow of blood from the heart (aortic stenosis), unstable angina (chest pain or tightness at rest or progressively increasing) or you have had a heart attack (myocardial infarction) one month ago or less and/or you require a pacemaker.
- taking other drugs for high blood pressure, such as beta-blockers, diuretics, ACE-inhibitors or angiotensin II receptor antagonists.
- You are currently pregnant or plan to become pregnant. Do not take this medicine whilst pregnant until you and your doctor have discussed the risks and benefits involved.
- You are currently breastfeeding or you plan to breast-feed. Do not take this medicine whilst breastfeeding until you and your doctor have discussed the risks and benefits involved.
- You are planning to have surgery or on an anaesthetic.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Taking other medicines
Some medicines may interact with lercanidipine. These include:
- cyclosporine
- ritonavir
- ketoconazole
- itraconazole
- erythromycin
- fluoxetine
- cimetidine
- phenytoin
- carbamazepine
- rifampicin
- amiodarone
- quinidine
- digoxin
- simvastatin
- metoprolol
- propranolol
If you are taking any of these you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with this medicine.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
The usual dose is one 10 mg tablet taken once daily, but may be increased to 20 mg once daily.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How to take it
Swallow the tablet whole with a glass of water.
When to take it
Take this medicine at about the same time each day, at least 15 minutes before a meal.
This medicine will have the best effect if it is taken at the same time each day. This will also help you remember when to take the tablets.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
This medicine helps control your condition but does not cure it therefore you must not stop taking it unless your doctor tells you to.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If you forget to take a dose but remember within 12 hours from when the dose was due, take it straight away, then continue as normal the next day. Otherwise skip that day's dose and take the next day's dose when it is due.
If you are not sure what to do, talk to your doctor or pharmacist.
Do not take a double dose to make up for missed doses. This may increase the chance of unwanted side effects.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints to help you remember.
If you have missed several doses, consult your doctor.
If you use too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency Department at your nearest hospital,
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Keep telephone numbers of these places/services handy.
If you take too much of this medicine, it may cause your blood pressure to become too low and you may feel your heart beat becomes irregular and faster. It may also lead to unconsciousness.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- You are about to be started on any new medicine
- You are pregnant or are planning to become pregnant
- You are breast-feeding or are planning to breast-feed
- You are about to have any blood tests
- You are going to have surgery or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Be sure to keep all of your doctor's appointments so that your progress can be checked.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to a child under the age of 18 years.
- Give this medicine to anyone else, even if their symptoms seem similar to yours
- Take your medicine to treat any other condition unless your doctor or pharmacist tells you to
- Stop taking your medicine, or change the dosage, without first checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how this medicine affects you.
This medicine generally does not affect your ability to drive a car or operate machinery. However, as with other medicines used to treat high blood pressure, some people may feel dizzy, light-headed or faint, especially when first taking this medicine or changing your dose.
Your doctor may also ask you to limit or stop your alcohol intake while taking medicines used to control your blood pressure as alcohol may increase these effects.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Grapefruit juice can increase the effects of some medicines including lercanidipine. If you are taking lercanidipine speak to your doctor or pharmacist before drinking grapefruit juice or changing your intake of grapefruit juice. As with some medicines, used to treat high blood pressure (such as lercanidipine), you should avoid drinking grapefruit juice as grapefruit juice may increase the effects of these medicines.
Possible side effects
Tell your doctor as soon as possible if you do not feel well while you are taking lercanidipine or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Tell your doctor if you notice any of the following:
- flushing
- swelling of the ankles, feet or lower legs
- headache
- dizziness
- gastrointestinal disturbances such as heartburn, nausea, abdominal pain or diarrhoea
- muscle weakness
- fatigue or sleepiness.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and are usually rare. You may need urgent medical attention or hospitalisation:
- angina (chest pain or tightness)
- increased heart beat or heart palpitations
- difficulty breathing
Other side effects not listed above may occur in some patients.
Allergic reactions
If you think you are having an allergic reaction to lercanidipine, tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing.
- swelling of the face, lips, tongue, or other parts of the body
- rash, itching or hives on the skin
- fainting
- hayfever-like symptoms.
Storage and disposal
Storage
Keep your medicine in the original packaging until it is time to take them. If you take your medicine out of the original packaging, it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 30°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or they have passed their expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
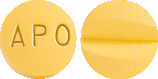
What APO-Lercanidipine looks like
APO-Lercanidipine is available in 10 mg or 20 mg tablets.
10 mg Tablets:
Yellow coloured, film coated, round shaped, biconvex tablets, engraved 'APO' on one side and score line on the other side.
Blister packs of 14, 28 and 30 tablets.
20 mg Tablets:
Pink coloured, film coated, round shaped, biconvex tablets, engraved 'APO' on one side and score line on the other side.
Blister packs of 14, 28 and 30 tablets.
APO-Lercanidipine also comes in bottles of 30, 100 & 500 tablets.
* Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each tablet contains 10 mg or 20 mg of lercanidipine (as lercanidipine hydrochloride) as the active ingredient.
It also contains the following inactive ingredients:
- Cellulose - microcrystalline
- lactose monohydrate
- croscarmellose sodium
- butylated hydroxytoluene
- magnesium stearate
- hypromellose
- macrogol 8000
- titanium dioxide
- purified talc
- yellow iron oxide (10 mg only)
- red iron oxide (20 mg only).
This medicine is gluten-free, sucrose-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
APO-Lercanidipine 10 mg tablets (blister pack): AUST R 163768
APO-Lercanidipine 10 mg tablets (bottle): AUST R 163765
APO-Lercanidipine 20 mg tablets (blister pack): AUST R 163769
APO-Lercanidipine 20 mg tablets (bottle): AUST R 163762
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park, NSW 2113
Australia
APO and APOTEX are registered trade marks of Apotex Inc.
This leaflet was prepared in July 2016



 More extensively, over 15,500 patients were treated with lercanidipine in clinical trials (including PMS studies) with doses from 2.5 mg daily up to 40 mg daily, and with treatment duration ranging from single dose up to more than 1 year. Adverse experiences which were not clearly drug related and which occurred in < 1% but ≥ 0.1% of patients are summarized according to organ system.
More extensively, over 15,500 patients were treated with lercanidipine in clinical trials (including PMS studies) with doses from 2.5 mg daily up to 40 mg daily, and with treatment duration ranging from single dose up to more than 1 year. Adverse experiences which were not clearly drug related and which occurred in < 1% but ≥ 0.1% of patients are summarized according to organ system.