SUMMARY CMI
APO-Ramipril
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using APO-Ramipril?
APO-Ramipril contains the active ingredient ramipril. APO-Ramipril is used to treat high blood pressure (hypertension), some heart conditions, such as heart failure after a heart attack, kidney problems in some patients.
For more information, see Section 1. Why am I using APO-Ramipril? in the full CMI.
2. What should I know before I use APO-Ramipril?
Do not use if you have ever had an allergic reaction to Ramipril or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use APO-Ramipril? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Ramipril and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use APO-Ramipril?
- Your doctor will tell you how many tablets you will need to take, depending on your conditions and whether you are taking any other medicines.
- If two tablets are prescribed, your doctor may want you to take both together or at different times.
More instructions can be found in Section 4. How do I use APO-Ramipril? in the full CMI.
5. What should I know while using APO-Ramipril?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines | Ramipril may cause dizziness, light-headedness, tiredness, or drowsiness in some people. Make sure you know how you react to ramipril before you drive a car, operate machinery, or do anything else that could be dangerous |
| Drinking alcohol | Alcohol may have effects when consumed while taking Ramipril. Your doctor may advise you to limit your alcohol intake. |
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using APO-Ramipril? in the full CMI.
6. Are there any side effects?
Side effects of Ramipril include feeling light-headed, dizzy or faint, persistent dry cough and/or ticking cough, headache, feeling sick, reflux, stomach pain or discomfort, diarrhoea, indigestion, muscle cramps.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
APO-Ramipril
Active ingredient(s): Ramipril
Consumer Medicine Information (CMI)
This leaflet provides important information about using Ramipril. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Ramipril.
Where to find information in this leaflet:
1. Why am I using APO-Ramipril?
2. What should I know before I use APO-Ramipril?
3. What if I am taking other medicines?
4. How do I use APO-Ramipril?
5. What should I know while using APO-Ramipril?
6. Are there any side effects?
7. Product details
1. Why am I using APO-Ramipril?
APO-Ramipril contains the active ingredient ramipril. Ramipril belongs to a group of medicines called angiotensin converting enzymes (ACE) inhibitors.
APO-Ramipril is used to treat high blood pressure (hypertension), some heart conditions, such as heart failure after a heart attack, kidney problems in some patients.
APO-Ramipril is also used to reduce the risk of cardiovascular problems and complications in patients aged 55 year or more with heart or blood vessels disease, or diabetes.
High blood pressure
Everyone has blood pressure. This pressure helps get your blood all around your body. Your blood pressure may be different at different times of the day and can be influenced by how busy or worried you are. You have high blood pressure when your blood pressure stays higher than is needed, even when you are calm and relaxed.
There are usually no symptoms of high blood pressure. The only way of knowing that you have high blood pressure is to have your blood pressure checked on a regular basis. Untreated high blood pressure can lead to serious health problems, including stroke, heart disease and kidney failure.
Heart failure after a heart attack
APO-Ramipril may be used after a heart attack, which occurs when one of the major blood vessels supplying blood to your heart becomes blocked. This means that your heart muscle cannot receive the oxygen it needs and becomes damaged. This may lead to further problems, such as heart failure, irregular heart rhythms and blood clots.
Heart failure means that the heart muscle is weak and cannot pump blood strongly enough to supply all the blood needed throughout the body. Heart failure is not the same as heart attack and does not mean that the heart stops. Heart failure may start off with no symptoms, but as the condition progresses, patients may feel short of breath or may get tired easily after light physical activity, such as walking. Some patients may wake up short of breath at night. Fluid may collect in different parts of the body, often first noticed as swollen ankles and feet.
Kidney problems
Some conditions, such as diabetes and high blood pressure, can lead to kidney problems. These problems develop slowly over several years. Good control of your blood sugar and blood pressure are important in keeping your kidneys healthy but may not always prevent kidney damage from occurring.
Prevention of Cardiovascular problems and complications
APO-Ramipril may be used to reduce the risk of some of the problems and complications that may arise in patients aged 55 years or more who have problems such as coronary artery disease (heart disease caused by poor blood flow in the blood vessels of the heart), peripheral vascular disease (poor circulation in the hands or feet) or stroke.
Patient with Diabetes
APO-Ramipril may also be used to reduce the risk of cardiovascular problems and complications in patients with diabetes aged 55 years or more who may be considered at risk because they have one or more additional risk factors (e.g. high blood pressure, high cholesterol levels, kidney problems, a current smoker or previous disease of the blood vessels).
2. What should I know before I use APO-Ramipril?
Warnings
Do not use APO-Ramipril if:
- you are allergic to ramipril, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- Have any of the following medical conditions:
- kidney problems, or are having dialysis (your doctor may give you ramipril because of your kidney problems)
- liver problems
- heart problems (your doctor may give you ramipril because of your heart problems)
- low blood pressure, which you may notice as dizziness or light-headedness.
- Low white blood cell counts
- diabetes (note: your doctor may give you ramipril because of your diabetes)
- high levels of potassium in your blood
- you are taking other medicines that have a risk of angioedema (swelling of the face, lips, tongue, throat, intestines, hands or feet)
- you are following a very low or very high salt diet
- you are dehydrated, or have had a recent bout of vomiting or diarrhoea
- Systemic Lupus Erythematosus (SLE), scleroderma or other autoimmune conditions
- you are planning to have surgery or an anaesthetic
- plan to become pregnant or breastfeed
Check with your doctor if you:
- have any other medical conditions.
- take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Ramipril and affect how it works.
- sacubitril/valsartan or other neprilysin inhibitors, used for heart failure
- other medicines used to treat high blood pressure, including those containing the active ingredient aliskiren
- diuretics, also known as fluid or water tablets
- lithium, used to treat mood swings and depression
- potassium supplements or potassium-containing salt substitutes
- non-steroidal anti-inflammatory drugs (NSAIDs), used to relieve pain and inflammation
- insulin and tablets used to treat diabetes
- heparin
- general anaesthetics
- medicines affecting blood cells (e.g. allopurinol, procainamide, corticosteroids, cancer treatments, immunosuppressants)
- temsirolimus
- if you are taking APO-Ramipril for high blood pressure, do not take any medicines (including the ones bought without a prescription) for appetite control, asthma, colds, coughs, hay fever or sinus problems unless you have discussed it with your doctor or pharmacist.
These medicines may be affected by APO-Ramipril or may affect how well it works. If you are taking any of these, you may need a different dose, or you may need to take different medicines. Your doctor will advise you.
Other medicines not listed above may also interact with APO-Ramipril.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect APO-Ramipril.
4. How do I use APO-Ramipril?
How much to take / use
- Your doctor will tell you how many tablets you will need to take, depending on your conditions and whether you are taking any other medicines.
- The usual dose is:
- For high blood pressure, 2.5mg to 10mg per day
- For heart failure, 5mg to 10mg per day
- For kidney problems, 1.25mg to 5mg per day
- For cardiovascular risk, 2.5mg to 10mg per day
If two tablets are prescribed, your doctor may want you totake both together and at different times.
When to take / use APO-Ramipril
APO-Ramipril should be used at about the same time each day.
Taking your tablets at the same time each day will have the best effect. It will also help you remember when to take the tablets. It does not matter if you take ramipril before or after food.
How to take APO-Ramipril
The tablets should be removed from the blister by applying gentle pressure, using thumb from one side of blister corner, carefully to avoid crumbling of tablets. The tablets should be swallowed whole with plenty of fluid.
If you forget to use APO-Ramipril
APO-Ramipril should be used regularly at the same time each day. If you miss your dose at the usual time, take it as soon as you remember and then go back to taking your medicine as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
This may increase the chance of you experiencing side effects.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you use too much APO-Ramipril
If you think that you have used too much Ramipril, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using APO-Ramipril?
Things you should do
Call your doctor straight away if you:
- If you are about to be started on any new medicine, tell your doctor, and pharmacist that you are taking APO-Ramipril.
- Tell your doctor immediately if you become pregnant.
- Make sure you drink enough water during exercise and hot weather when you are taking ramipril, especially if you sweat a lot.
- If you do not drink enough water while taking ramipril, you may feel faint, light-headed or sick. This is because your blood pressure is dropping suddenly. If you continue to feel unwell, tell your doctor.
- If you have excess vomiting or diarrhoea while taking ramipril, tell your doctor.
- You may lose too much water and salt and your blood pressure may drop too much.
- Tell your doctor immediately if you feel light-headed or dizzy after taking your first dose of ramipril, or when your dose is increased.
- If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking ramipril as your blood pressure may drop suddenly.
- APO-Ramipril may also affect other medicines used during surgery.
- If you are about to have any blood tests, tell your doctor that you are taking ramipril as it may interfere with the results of some tests.
- Have your blood pressure checked when your doctor says, to make sure ramipril is working.
- Your doctor may do some test to make sure the medicine is working and to prevent side effects.
- Keep all of your doctor's appointments so that your progress can be checked.
- Your doctor may occasionally do a blood test to check your potassium levels and see how your kidneys are working.
Remind any doctor, dentist, or pharmacist you visit that you are using Ramipril.
Things you should not do
- Do not stop using this medicine suddenly or change the dosage, without first checking with your doctor.
- Do not give this medicine to anyone else, even if they have the same condition as you.
- Do not take your medicine to treat any other complaint unless your doctor tells you to.
Things to be careful of
- If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
- Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
- Diet - eat a healthy low-fat diet which includes plenty of fresh vegetables, fruit, bread, cereals and fish. Eat less fat and sugar.
- Exercise - regular exercise helps to reduce blood pressure and helps get the heart fitter, but it is important not to overdo it.
- Walking is good exercise but try to find a route that is reasonably flat. Before starting any exercise, ask your doctor about the best kind of exercise programme for you.
- Salt - your doctor may advise you to watch the amount of salt in your diet. To reduce your salt intake, you should avoid using salt in cooking or at the table.
- Smoking - your doctor may advise you to stop, or at least cut down, smoking.
- Weight - your doctor may suggest losing some weight to help lower your blood pressure and help lessen the amount of work your heart must do. Some people may need a dietician's help to lose weight.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how APO-Ramipril affects you.
Ramipril may cause dizziness, light-headedness, tiredness, or drowsiness in some people. Make sure you know how you react to ramipril before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy or light-headed. If you drink alcohol, dizziness or light-headedness may be worse.
Drinking alcohol
Tell your doctor if you drink alcohol.
Alcohol may have effects when consumed while taking APO-Ramipril. Your doctor may advise you to limit your alcohol intake.
Looking after your medicine
- Keep your medicine in its pack until it is time to take it.
- If you take your medicine out of its pack it may not keep well.
- Keep your medicine in a cool dry place where the temperature is below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine (as relevant)
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine leftover.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Very Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these very serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What APO-Ramipril contains
| Active ingredient (main ingredient) | Ramipril |
| Other ingredients (inactive ingredients) | hypromellose microcrystalline cellulose pregelatinised maize starch iron oxide red (2.5 mg tablet only) iron oxide yellow (5 mg and 10mg tablet only) sodium stearyl fumarate |
| Potential allergens | N/A |
This medicine does not contain gluten, lactose, sucrose, tartrazine, or any other azo dyes.
Do not take this medicine if you are allergic to any of these ingredients.
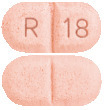
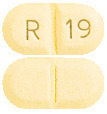
What APO-Ramipril looks like
2.5 mg Tablet:
Pink to red mottled, oblong tablet with R and 18 on either side of score line on one side and score line on the other side. AUST R 231161
5 mg Tablet:
Light yellow to yellow mottled, oblong tablet with R and 19 on either side of score line on one side and score line on the other side. AUST R 231163
10 mg Tablet:
Light yellow to yellow mottled, oblong tablet with R and 10 on either side of score line on one side and score line on the other side. AUST R 231162
Available as blister pack of 30 tablets.
Who distributes APO-Ramipril
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Australia
This leaflet was prepared in January 2024
Published by MIMS March 2024


 The improvement in these key endpoints was observed to increase with time, to be maintained long term and to apply to both hypertensive and non-hypertensive patients. A delay of approximately three months was seen prior to detection of the beneficial effects of ramipril, suggesting the value of early treatment.
The improvement in these key endpoints was observed to increase with time, to be maintained long term and to apply to both hypertensive and non-hypertensive patients. A delay of approximately three months was seen prior to detection of the beneficial effects of ramipril, suggesting the value of early treatment.
 The results of the HOPE study in terms of the pre-specified secondary endpoints for the whole population (ITT - Intention to Treat) are presented in Table 4.
The results of the HOPE study in terms of the pre-specified secondary endpoints for the whole population (ITT - Intention to Treat) are presented in Table 4. The results of the HOPE study in terms of the pre-specified secondary endpoints for those patients with diabetes are presented in Table 5.
The results of the HOPE study in terms of the pre-specified secondary endpoints for those patients with diabetes are presented in Table 5.
 Ramipril has 5 chiral centres, with S-configuration in all 5 asymmetric carbon atoms.
Ramipril has 5 chiral centres, with S-configuration in all 5 asymmetric carbon atoms.