SUMMARY CMI
Cabaser®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Cabaser?
Cabaser contains the active ingredient cabergoline. Cabaser is used in the management of the signs and symptoms of Parkinson's disease. For more information, see Section 1. Why am I using Cabaser? in the full CMI.
2. What should I know before I use Cabaser?
Cabaser may cause heart valve problems or may affect your lung and kidney function. Before starting treatment with Cabaser your doctor will need to do some tests, to detect any underlying heart, lung or kidney disease.
Do not take Cabaser if you have or have had any scarring or thickening of the lungs with shortness of breath; any evidence of heart valve disorder; any swelling or inflammation around the heart or lungs; or any abnormal formation of scar tissue outside the stomach wall.
Do not take if you have an allergy to any medicine containing cabergoline, any other ergot alkaloid or any of the ingredients listed. For more information, see Section 2. What should I know before I use Cabaser? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with cabergoline and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Cabaser?
The dose varies from patient to patient. Your doctor will tell you how many tablets to take.
The usual starting dose is half to one 1 mg tablet (0.5 mg to 1 mg) a day. Your doctor will gradually increase your dose over several weeks. The recommended maintenance dose is 2 mg to 3 mg a day.
More instructions can be found in Section 4. How do I use Cabaser? in the full CMI.
5. What should I know while using Cabaser?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Cabaser? in the full CMI.
6. Are there any side effects?
Some common side effects include unusual sleepiness, abnormal movements, increased sweating, diarrhoea, constipation, lower stomach pain, nausea, vomiting, dizziness, fainting, headache, weakness, excessive tiredness, leg cramps, back pain, unusual hair loss or thinning, aggressive behaviour, changes in behaviour. For more serious side effects and further information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
Cabaser® (cab-a-sar)
Active ingredient(s): cabergoline (ca-ber-go-lean)
Consumer Medicine Information (CMI)
This leaflet provides important information about using Cabaser. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Cabaser.
Where to find information in this leaflet:
1. Why am I using Cabaser?
2. What should I know before I use Cabaser?
3. What if I am taking other medicines?
4. How do I use Cabaser?
5. What should I know while using Cabaser?
6. Are there any side effects?
7. Product details
1. Why am I using Cabaser?
Cabaser contains the active ingredient cabergoline.
Cabaser is used in the management of the signs and symptoms of Parkinson's disease. This is a disease of the brain affecting movement. The main symptoms are tremor, rigid posture, slow movements and a shuffling, unbalanced walk. Cabaser helps to reduce these symptoms and to improve your ability to do your normal, everyday tasks.
Parkinson's disease is caused by the brain not making enough of a chemical called dopamine. Dopamine helps the brain to control muscle movement.
Cabaser belongs to a group of medicines called dopamine agonists. It works by increasing the effects of dopamine.
2. What should I know before I use Cabaser?
Warnings
Cabaser may cause heart valve problems or may affect your lung and kidney function.
Before starting treatment with Cabaser your doctor will need to do some tests, to detect any underlying heart, lung or kidney disease.
These tests include chest x-rays, physical examinations, blood tests and heart monitoring.
Your doctor will repeat these tests regularly while you are on Cabaser.
While taking Cabaser be sure to tell your doctor about anything that is making you feel unwell, such as difficulty breathing, chest pain or swelling of your hands or feet.
Do not take Cabaser if you have or have had:
- any scarring or thickening of the lungs with shortness of breath
- any evidence of heart valve disorder
- any swelling or inflammation around the heart or lungs
- any abnormal formation of scar tissue outside the stomach wall.
- an allergy to any medicine containing cabergoline, any other ergot alkaloid or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine. Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not give this medicine to a child.
Safety and effectiveness in children have not been established.
Check with your doctor if you:
- have or have had any of the following medical conditions:
- liver problems
- lung disease or problems with your breathing
- heart problems including chest pain, a recent heart attack
- Raynaud's syndrome (problems with the circulation in the fingers and toes causing the skin colour to turn pale or blue)
- stomach ulcer
- bleeding from your stomach and/or gut
- mental illness
- low blood pressure. - take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or plan to become pregnant.
Your doctor will discuss with you the risks and benefits involved.
It is recommended that women who plan to become pregnant stop taking Cabaser at least one month before becoming pregnant.
Tell your doctor if you are breastfeeding or plan to breastfeed.
Your doctor will discuss with you the risks and benefits involved. It is possible that Cabaser will prevent the production of milk.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by Cabaser or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
Tell your doctor or pharmacist if you are taking any of the following:
- medicines used to lower your blood pressure
- other medicines used for Parkinson's disease
- medicines used to treat mental illness
- metoclopramide, a medicine used to treat nausea
- macrolide antibiotics such as erythromycin.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Cabaser.
4. How do I use Cabaser?
How much to take
The dose varies from patient to patient.
Your doctor will tell you how many tablets to take. The usual starting dose is half to one 1 mg tablet (0.5 mg to 1 mg) a day. Your doctor will gradually increase your dose over several weeks. The recommended maintenance dose is 2 mg to 3 mg a day.
Follow all instructions from your doctor and pharmacist carefully.
How to take it
Swallow the tablets whole with a full glass of water.
If your doctor has prescribed half a tablet, break the tablet in half along the score line.
When to take Cabaser
Take your medicine once a day, preferably with meals.
Taking it with food will lessen any side effects you may experience.
Take your medicine at about the same time each day.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
If you are not sure of how to take Cabaser, ask your doctor or pharmacist.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If you forget to take one or more of your Cabaser tablets, take your next tablet at the normal time it is due.
Do not take a double dose to make up for the dose that you missed.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much Cabaser
If you think that you have taken too much Cabaser, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of an overdose may include:
- nausea
- vomiting
- stomach complaints
- feeling dizzy
- confusion
- hallucinations.
Have the Cabaser bottle or box or this leaflet available to give details if needed.
5. What should I know while using Cabaser?
Things you should do
Follow your doctor's requests for tests and report anything unusual to your doctor such as difficulty in breathing, chest pain or swelling of your hands or feet.
Keep all of your doctor's appointments so that your progress can be checked.
It is important that your doctor does some tests every 6 to 12 months while you are taking Cabaser to make sure the medicine is working and to prevent unwanted side effects. These tests may include chest x-rays, physical examinations, blood tests and heart monitoring.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Cabaser.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you should not do
Do not take Cabaser to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor.
If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things to be careful of
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
See your doctor if you notice changes in your behaviour that result in a strong desire to either gamble, shop, eat or use medicines to excess, or you notice an increase in your sex drive.
Such compulsive behaviours have been seen with some medicines used to treat Parkinson's disease, including Cabaser.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Cabaser affects you.
In some people, Cabaser may cause dizziness, light headedness or irregular movements especially during the first days of taking it. Some people may also experience sleepiness and/or sudden onset of sleep. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Be careful when drinking alcohol while taking Cabaser.
The effect of drinking alcohol while taking Cabaser is not known.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Store your tablets in the bottle until it is time to take them.
If you take the tablets out of the bottle they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Do not use this medicine after the expiry date.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Common side effects
| Common side effects | What to do |
| Speak to your doctor if you have any of these common side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Tell your doctor as soon as possible. You may require medical attention. |
Very serious side effects
| Very serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. You may need urgent medical attention or hospitalisation. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Some of these side effects (for example, changes in blood pressure, and certain heart, lung and liver conditions) can only be found when your doctor does tests from time to time to check your progress.
Your doctor will do some tests every 6 to 12 months while you are taking Cabaser to help prevent unwanted side effects. For example, chest x-rays, physical examinations, blood tests and heart monitoring. Such tests can detect leaky and/or narrowed valves of the heart and any scarring or thickening of the lungs.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Cabaser contains
| Active ingredient (main ingredient) | Cabaser 1 mg - 1 mg cabergoline per tablet Cabaser 2 mg - 2 mg cabergoline per tablet |
| Other ingredients (inactive ingredients) | lactose leucine |
Do not take this medicine if you are allergic to any of these ingredients.
What Cabaser looks like
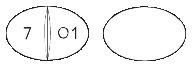
Cabaser 1 mg - white, oval, both sides concave tablets, one side scored and engraved '7' on the left of the break-line and '01' on the right of it.
AUST R 57360
Cabaser 2 mg - white, oval, both sides concave tablets, one side scored and engraved '7' on the left of the break-line and '02' on the right of it.
AUST R 57367
All Cabaser tablets have a score mark which allows the tablet to be broken in half.
Cabaser tablets come in bottles with child-resistant caps and enclosed in an outer cardboard carton.
Each pack contains 30 tablets.
Who distributes Cabaser
Pfizer Australia Pty Ltd
Sydney NSW
Toll Free Number: 1800 675 229
www.pfizermedicalinformation.com.au
This leaflet was prepared in November 2022.
® Registered Trademark
© Pfizer Australia Pty Ltd 2022
Published by MIMS February 2023