SUMMARY CMI
Cabometyx®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
▼ This medicine is new or being used differently. Please report side effects. See the full CMI for further details.
1. Why am I using CABOMETYX?
CABOMETYX contains the active ingredient cabozantinib. CABOMETYX is used to treat patients with advanced kidney cancer (advanced renal cell carcinoma), liver cancer, or a type of thyroid cancer called differentiated thyroid cancer.
For more information, see Section 1. Why am I using CABOMETYX? in the full CMI.
2. What should I know before I use CABOMETYX?
Do not use if you have ever had an allergic reaction to cabozantinib or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use CABOMETYX? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with CABOMETYX and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use CABOMETYX?
- The usual dose is 60 mg taken once a day. Your doctor will decide on the right dose for you.
- CABOMETYX should not be taken with food. Grapefruit juice should be avoided while using this medicine.
More instructions can be found in Section 4. How do I use CABOMETYX? in the full CMI.
5. What should I know while using CABOMETYX?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using CABOMETYX? in the full CMI.
6. Are there any side effects?
Like all medicines, CABOMETYX can cause side effects, although not everybody gets them. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
Tell your doctor straight away if you notice any of the following side effects, you may need urgent medical attention:
- pain in the abdomen (belly), feeling sick, vomiting, constipation, fever. These may be signs of a hole that develops in your stomach or intestine (common).
- vomiting blood, black stools, bloody urine, headache, coughing up blood. These may be signs of severe bleeding inside your body (very common).
- swelling, pain in your hands and feet, or shortness of breath. These may be signs of blood clots or oedema (common).
- feeling drowsy or confused, loss of consciousness. These may be signs of liver problems (common).
- coughing, difficulty breathing. These may be signs of a lung infection (common).
- kidney failure including abrupt loss of kidney function (common).
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
FULL CMI
Cabometyx®
Active ingredient(s): cabozantinib
Consumer Medicine Information (CMI)
This leaflet provides important information about using CABOMETYX. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using CABOMETYX.
Where to find information in this leaflet:
1. Why am I using CABOMETYX?
2. What should I know before I use CABOMETYX?
3. What if I am taking other medicines?
4. How do I use CABOMETYX?
5. What should I know while using CABOMETYX?
6. Are there any side effects?
7. Product details
1. Why am I using CABOMETYX?
CABOMETYX contains the active ingredient cabozantinib (as cabozantinib (S)-malate). CABOMETYX is a multi-kinase inhibitor.
It works by blocking the action of proteins called receptor tyrosine kinases (RTKs), which are involved in the growth of cells and the development of new blood vessels that supply them. These proteins can be present in high amounts in cancer cells, and by blocking their action CABOMETYX can slow down the rate at which the tumour grows and help to cut off the blood supply that the cancer needs.
CABOMETYX is used to treat:
- advanced stages of a type of kidney cancer called renal cell carcinoma (RCC)
- liver cancer in adults who have been previously treated with a specific anticancer medicine (sorafenib)
- a type of thyroid cancer called differentiated thyroid cancer (DTC), in adults and children 12 years of age and older, that has spread (locally advanced or metastatic), and,
- has progressed after treatment with a medicine called VEGFR-targeted treatment, and,
- when the DTC can no longer be treated with radioactive iodine, or patients are not able to receive radioactive iodine treatment.
CABOMETYX may also be given in combination with another medicine called nivolumab to treat advanced kidney cancer (RCC). It is important that you also read the Consumer Medicine Information of nivolumab.
2. What should I know before I use CABOMETYX?
Warnings
Do not use CABOMETYX if:
- you are allergic to cabozantinib, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Check with your doctor if you:
- have intolerance to some sugars. The tablets contain lactose.
- have high blood pressure.
- have, or have had, an aneurysm (enlargement and weakening of a blood vessel wall) or a tear in a blood vessel wall.
- have diarrhoea.
- have a recent history of significant bleeding.
- have had surgery within the last month (or if surgical procedures are planned), including dental surgery.
- have inflammatory bowel disease (for example, Crohn's disease or ulcerative colitis, diverticulitis, or appendicitis).
- have a recent history of blood clot in the leg, stroke, or heart attack.
- have liver or kidney disease.
- have a pre-existing heart condition, slow heart rate or are taking medicine to prevent abnormal heart rhythm.
- take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
You should avoid becoming pregnant while being treated with CABOMETYX.
CABOMETYX should not be taken during pregnancy. Your doctor will discuss the risks with you.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
You should not breastfeed while taking CABOMETYX and for at least 4 months after treatment has finished, as cabozantinib and/or its metabolites may be excreted in breast milk and be harmful to your child.
If you or your partner could become pregnant, you must use a safe and effective form of contraception (such as a condom or coil) to avoid becoming pregnant while you are being treated with CABOMETYX. You should also do this for at least 4 months after stopping treatment. Discuss with your doctor what may be appropriate contraception for you.
Tell your doctor if you are taking oral contraceptives.
If you take CABOMETYX whilst using oral contraceptives, the oral contraceptives may be ineffective.
Tell your doctor if you or your partner plan to become pregnant in the future.
CABOMETYX may affect your fertility.
Children and Teenagers
- CABOMETYX is not recommended for children or adolescents for treatment of liver or kidney cancer.
- CABOMETYX can be used to treat children and adolescents 12 years of age or older with thyroid cancer.
- It is not known if CABOMETYX is safe and effective in children younger than 12 years of age.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with CABOMETYX and affect how it works.
You should tell your doctor about every medicine, but in particular if you are taking:
- Medicines that treat fungal infections, such as itraconazole, ketoconazole, and posaconazole.
- Medicines used to treat bacterial infections (antibiotics) such as erythromycin, clarithromycin, and rifampicin.
- Allergy medicines such as fexofenadine.
- Medicines to treat angina pectoris (chest pain owing to inadequate blood supply to the heart) such as ranolazine.
- Medicines used to treat epilepsy or fits such as phenytoin, carbamazepine, and phenobarbital.
- Herbal preparations containing St. John's Wort (Hypericum perforatum), sometimes used for treating depression or depression-related conditions such as anxiety.
- Medicines used to thin the blood, such as warfarin and dabigatran etexilate.
- Medicines to treat high blood pressure or other heart conditions, such as ambrisentan, digoxin, and tolvaptan.
- Medicines for diabetes, such as saxagliptin and sitagliptin.
- Medicines used to treat gout, such as colchicine.
- Medicines used to treat HIV or AIDS, such as efavirenz, ritonavir, maraviroc and emtricitabine.
- Medicines used to prevent transplant rejection (ciclosporin) and ciclosporin-based regimens in rheumatoid arthritis and psoriasis.
- Medicines used for contraception such as oral contraceptives, as they may be ineffective whilst using CABOMETYX.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect CABOMETYX.
4. How do I use CABOMETYX?
How much to take
- The usual dose of CABOMETYX is 60 mg taken once a day.
- When CABOMETYX is given in combination with nivolumab for the treatment of advanced kidney cancer, the recommended dose of CABOMETYX is 40 mg once a day.
- Your doctor will decide on the right dose for you.
- Follow the instructions provided and use CABOMETYX until your doctor tells you to stop.
How to take CABOMETYX
- Swallow the tablet whole with a full glass of water.
- Do not crush the tablets.
- CABOMETYX should not be taken with food. You should not eat anything for at least 2 hours before taking CABOMETYX and for 1 hour after taking the medicine.
- Avoid consuming grapefruit juice or grapefruit-containing products for as long as you are using this medicine, as they may increase the levels of CABOMETYX in your blood.
When to take CABOMETYX
- CABOMETYX should be taken at about the same time each day.
- When CABOMETYX is given in combination with nivolumab, you will first be given nivolumab followed by CABOMETYX.
- Continue taking your medicine for as long as your doctor tells you.
If you forget to use CABOMETYX
CABOMETYX should be used regularly at the same time each day. If there are still 12 hours or more before your next dose is due, then take the missed dose as soon as you remember.
If your next dose is due in less than 12 hours, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much CABOMETYX
If you think that you have used too much CABOMETYX, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using CABOMETYX?
Things you should do
Call your doctor straight away if you:
- Experience any of the symptoms under Serious Side Effects in Section 6.
Remind any doctor or pharmacist you visit that you are using CABOMETYX.
You should also tell your dentist that you are taking CABOMETYX. It is important for you to practice good mouth care during treatment with CABOMETYX.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking CABOMETYX. It may affect other medicines used during surgery.
If you become pregnant while taking CABOMETYX, tell your doctor immediately. Do not stop treatment without first discussing it with your doctor.
Keep all of your doctor's appointments so that your progress can be checked.
Things you should not do
- Do not stop using this medicine suddenly.
- Do not take CABOMETYX to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not stop taking your medicine or lower the dosage without checking with your doctor.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how CABOMETYX affects you.
Treatment with CABOMETYX may make you feel tired or weak and can affect your ability to drive or operate machines.
Looking after your medicine
- Keep your medicine in the original container.
- If you take it out of its original container it may not keep well.
- Store your tablets in a cool, dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Serious side effects
| Serious side effects | What to do |
Signs of severe or uncontrollable bleeding inside your body:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Signs of lung infection:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Less serious side effects
| Less serious side effects | What to do |
Infection related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Breathing or respiratory system related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Muscle or bone related:
| Speak to your doctor if you have any of these less serious side effects and they worry you |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What CABOMETYX contains
| Active ingredient (main ingredient) | cabozantinib (as cabozantinib (S) -malate) |
| Other ingredients (inactive ingredients) | microcrystalline cellulose lactose hyprolose croscarmellose sodium colloidal anhydrous silica magnesium stearate hypromellose titanium dioxide triacetin iron oxide yellow |
| Potential allergens | lactose |
Do not take this medicine if you are allergic to any of these ingredients.
This medicine does not contain gluten, tartrazine or any other azo dyes.
What CABOMETYX looks like
CABOMETYX 20 mg film-coated tablets are yellow, round with no score, and identified with "XL" on one side and "20" on the other side. (AUST R 283800)
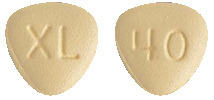
CABOMETYX 40 mg film-coated tablets are yellow, triangle shaped with no score, and identified with "XL" on one side and "40" on the other side. (AUST R 283801)
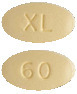
CABOMETYX 60 mg film-coated tablets are yellow, oval shaped with no score, and identified with "XL" on one side and "60" on the other side. (AUST R 283799)
CABOMETYX tablets are available in a plastic bottle with 30 tablets.
The bottle contains three silica gel desiccant canisters. Keep the canisters in the bottle and do not swallow the desiccant canisters.
Australian Sponsor of CABOMETYX
Ipsen Pty Ltd
Level 5, 627 Chapel Street
South Yarra VIC 3141
Cabometyx® is a registered trademark of Exelixis, Inc., licensed to Ipsen Pharma S.A.S.
This leaflet was prepared in April 2023.
Published by MIMS June 2023













 Efficacy results from the primary analysis (minimum follow up 10.6 months) are shown in Table 8.
Efficacy results from the primary analysis (minimum follow up 10.6 months) are shown in Table 8. PFS, OS, ORR benefit was observed in the cabozantinib in combination with nivolumab arm vs. sunitinib regardless of tumour PD L1 expression.
PFS, OS, ORR benefit was observed in the cabozantinib in combination with nivolumab arm vs. sunitinib regardless of tumour PD L1 expression.

 The incidence of systemic non-radiation and local liver-directed systemic non-protocol anticancer therapy (NPACT) was 26% in the cabozantinib arm and 33% in the placebo arm. Subjects receiving these therapies had to discontinue study treatment. An exploratory OS analysis censoring for the use of NPACT supported the primary analysis: the HR, adjusted for stratification factors (per IxRS), was 0.66 (95% CI: 0.52, 0.84; stratified logrank p-value = 0.0005). The Kaplan-Meier estimates for median duration of OS were 11.1 months in the cabozantinib arm versus 6.9 months in the placebo arm, an estimated 4.2-month difference in the medians.
The incidence of systemic non-radiation and local liver-directed systemic non-protocol anticancer therapy (NPACT) was 26% in the cabozantinib arm and 33% in the placebo arm. Subjects receiving these therapies had to discontinue study treatment. An exploratory OS analysis censoring for the use of NPACT supported the primary analysis: the HR, adjusted for stratification factors (per IxRS), was 0.66 (95% CI: 0.52, 0.84; stratified logrank p-value = 0.0005). The Kaplan-Meier estimates for median duration of OS were 11.1 months in the cabozantinib arm versus 6.9 months in the placebo arm, an estimated 4.2-month difference in the medians. Exploratory analyses of PFS in the ITT population have also shown consistent results in favour of Cabometyx compared to placebo across different subgroups according to prior lenvatinib, prior sorafenib, number of prior VEGFR, age (≤ 65 years or > 65 years), histology type (papillary or follicular).
Exploratory analyses of PFS in the ITT population have also shown consistent results in favour of Cabometyx compared to placebo across different subgroups according to prior lenvatinib, prior sorafenib, number of prior VEGFR, age (≤ 65 years or > 65 years), histology type (papillary or follicular).

