SUMMARY CMI
CADUET®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using CADUET?
CADUET contains the active ingredient amlodipine besilate and atorvastatin calcium trihydrate. CADUET is used to treat high blood pressure and high cholesterol (fat in the blood). CADUET can also be used for angina (a certain type of chest pain). CADUET can be used in people who have high blood pressure and coronary heart disease (CHD) or who are at risk of CHD (for example, if they have diabetes, a history of stroke, or small blood vessel disease). In these people, CADUET is used to help reduce the risk of having a heart attack or stroke.
For more information, see Section 1. Why am I using CADUET? in the full CMI.
2. What should I know before I use CADUET?
Do not use if you have ever had an allergic reaction to CADUET or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use CADUET? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with CADUET and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use CADUET?
- CADUET is taken once a day. Your doctor will decide which strength is suitable for you.
More instructions can be found in Section 4. How do I use CADUET? in the full CMI.
5. What should I know while using CADUET?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using CADUET? in the full CMI.
6. Are there any side effects?
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Ask your doctor or pharmacist for advice if you experience any of the following common side effects and they worry you, headache, flushing, dizziness, tiredness or weakness, drowsiness or sleepiness, stomach pain or nausea.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
CADUET®
Active ingredients: (amlodipine besilate and atorvastatin calcium trihydrate)
Consumer Medicine Information (CMI)
This leaflet provides important information about using CADUET. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using CADUET.
Where to find information in this leaflet:
1. Why am I using CADUET?
2. What should I know before I use CADUET?
3. What if I am taking other medicines?
4. How do I use CADUET?
5. What should I know while using CADUET?
6. Are there any side effects?
7. Product details
1. Why am I using CADUET?
CADUET contains the active ingredients amlodipine and atorvastatin.
CADUET is used to treat high blood pressure and high cholesterol (fat in the blood). CADUET can also be used for angina (a certain type of chest pain). CADUET can be used in people who have high blood pressure and coronary heart disease (CHD) or who are at risk of CHD (for example, if they have diabetes, a history of stroke, or small blood vessel disease). In these people, CADUET is used to help reduce the risk of having a heart attack or stroke.
Ask your doctor if you have any questions about why
Caduet has been prescribed for you.
Your doctor may have prescribed it for another reason.
Caduet is not addictive.
This medicine is available only with a doctor's prescription.
How CADUET works
Amlodipine belongs to a group of medicines called calcium channel blockers. They work by relaxing your blood vessels, making it easier for your heart to pump blood around the body and help increase the supply of blood and oxygen to your heart. Calcium channel blockers do not change the amount of calcium in your blood or bones.
Atorvastatin belongs to a group of medicines called statins. It works by reducing the amount of cholesterol made by the liver. It reduces the 'bad' cholesterol and can raise the 'good' cholesterol. Atorvastatin also helps to protect you from a heart attack or stroke.
What is high blood pressure?
There are usually no symptoms of high blood pressure (hypertension). The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. If high blood pressure is not treated it can lead to serious health problems.
What is cholesterol?
Everyone has cholesterol in their blood. It is a type of blood fat needed by the body for things such as building cell lining, making bile acids (which help to digest food) and some hormones. However, too much cholesterol can be a problem.
Cholesterol is present in many foods and is also made in your body by the liver. If your body makes too much cholesterol or you take too much cholesterol in your diet, then your level becomes too high.
High cholesterol is more likely to occur with certain diseases or if you have a family history of high cholesterol.
There are different types of cholesterol. LDL, or low-density lipoprotein, is the 'bad' cholesterol that can block your blood vessels. HDL, or high-density lipoprotein, is the 'good' cholesterol that is thought to remove the 'bad' cholesterol from the blood vessels.
When you have high levels of 'bad' cholesterol in your blood, it may begin to 'stick' to the inside of your blood vessels instead of being carried to the parts of the body where it is needed. Over time, this can form hard areas, also called plaque, on the walls of your blood vessels, making it more difficult for the blood to flow. Sometimes, the plaque can detach from the vessel wall and float in the bloodstream; it can then reach a smaller vessel and completely block it. This blocking of your blood vessels can lead to several types of blood vessel disease, heart attack, angina and stroke.
There is another type of blood fat called triglyceride that is a source of energy. However, high levels of triglyceride can be associated with a low level of 'good' cholesterol and may increase your risk of heart disease.
In some patients, CADUET is used to treat high cholesterol and high triglycerides together.
In most people, there are no symptoms of abnormal cholesterol or triglyceride levels. Your doctor can measure your levels with a simple blood test.
What is angina?
Angina is a pain or uncomfortable feeling in the chest, often spreading to the arms or neck, and sometimes to the shoulders and back. The pain of angina is due to a shortage of oxygen to the heart. CADUET is used to treat chronic angina.
2. What should I know before I use CADUET?
Warnings
Do not take CADUET if you:
- have an allergy to any medicine containing amlodipine
- have an allergy to any medicine containing atorvastatin
- have any allergy to any of the ingredients listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue, or other parts of the body
- rash, itching or hives on the skin
Always check the ingredients to make sure you can use this medicine. - have active liver disease.
- are pregnant or intend to become pregnant
- If you are a woman of childbearing age and are taking this medicine, use a proven method of birth control to avoid pregnancy
- This medicine may affect your unborn developing baby if you take it during pregnancy - are breastfeeding or intend to breastfeed
- This medicine passes into breast milk and may affect your baby - are taking the antibiotic fusidic acid hemihydrate which is used to treat infections
- are taking the antivirals glecaprevir/pibrentasvir for the treatment of Hepatitis C.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking CADUET, talk to your doctor.
Check with your doctor if you:
- have or have had any of the following medical conditions:
- heart problems, including heart failure
- liver problems
- kidney problems
- muscle pain, tenderness or weakness from other
- medicines used to treat cholesterol or triglycerides
- vision problems, eye muscle weakness and drooping eyelid
- breathing problems
- a type of stroke called a haemorrhagic stroke or a type of stroke called a lacunar stroke.
If you have had one of these strokes before, this medicine may increase the risk of you having a haemorrhagic stroke. - take any medicines for any other condition
- have allergies to any other medicines, foods, preservatives or dyes.
Your doctor will ask you to have your liver function tested before you start to take CADUET.
Tell your doctor if you are consuming alcohol regularly.
If you have not told your doctor about any of the above, tell him/her before you start taking Caduet.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Do not take this medicine if you are pregnant or intend to become pregnant.
If you are a woman of child-bearing age and are taking this medicine, use a proven method of birth control to avoid pregnancy.
This medicine may affect your unborn developing baby if you take it during pregnancy.
Do not take this medicine if you are breastfeeding or intend to breastfeed.
This medicine passes into breast milk and may affect your baby.
Use in the elderly
If you are 65 years or older, you should be especially careful while taking CADUET. Report any side effects promptly to your doctor.
Some people in this age group may be more likely to experience side effects such as swelling of the feet and ankles, muscle cramps and dizziness.
Use in Children
There is not enough information to recommend the use of this medicine in children.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with CADUET and affect how it works.
Tell your doctor or pharmacist if you are taking:
- other calcium channel blockers. These medicines include amlodipine which is also in CADUET; other calcium channel blockers include medicines with the active ingredient felodipine or nifedipine.
- other statins. These medicines include atorvastatin which is also in CADUET. Other statins include medicines with the active ingredient fluvastatin, pravastatin, rosuvastatin, simvastatin or simvastatin containing medicines.
Check with your doctor or pharmacist if you are unsure.
Some medicines may be affected by Caduet or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
Tell your doctor or pharmacist if you are taking any of the following:
- digoxin, a medicine used to treat some heart problems
- the antibiotics erythromycin, clarithromycin, rifampicin or fusidic acid hemihydrate
- phenytoin, a medicine used to treat epilepsy (seizures)
- oral contraceptives for birth control
- other medicines to treat high cholesterol or triglycerides (fats)
- other medicines to treat high blood pressure
- some medicines to treat low potassium
- ciclosporin, tacrolimus, sirolimus or everolimus, medicines used to suppress the immune system
- temsirolimus, a medicine used to treat kidney cancer
- some medicines used to treat some fungal infections, such as ketoconazole or itraconazole
- protease inhibitors for the treatment of HIV infection and/or Hepatitis C, such as efavirenz, fosamprenavir, ritonavir, boceprevir, telaprevir, tipranavir/ritonavir, elbasvir/grazoprevir and simprevir
- HCV non structural protein 5A (NS5A)/ 5B (NS5B) inhibitors such as daclatasvir and ledipasvir
- letermovir
- diltiazem, a medicine used to treat angina
- antacids, medicines used to treat reflux or ulcers
- spironolactone, a medicine used to treat high blood pressure and certain types of swelling
- vitamin B3
- colchicine, a medicine used to treat a disease with painful swollen joints caused by uric acid crystals
- ticagrelor, a medicine used to prevent blood clots.
It is also possible that CADUET may be affected by some medicines used to treat heart palpitations (or arrhythmias) and St John's Wort.
Your doctor and pharmacist have more information on medicines to be careful with or to avoid while taking Caduet.
If you have not told your doctor about any of the above, tell him/her before you start taking Caduet.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect CADUET.
4. How do I take CADUET?
How much to take
- CADUET is taken once a day.
- Your doctor will decide which strength is suitable for you. This will depend on your blood pressure, cholesterol and triglyceride levels.
Your doctor may need to adjust your dose after your blood pressure and the fat levels in your blood have been checked. It is important that you keep your appointments to have these tests done.
When to take CADUET
- Take your medicine once a day at about the same time each day, either morning or evening.
Taking it at the same time each day will have the best effect. It will also help you to remember when to take it. - CADUET can be taken with or without food.
How to take CADUET
- Swallow the tablet whole with a full glass of water.
- Do not chew or crush the tablets.
- Take CADUET exactly as your doctor has prescribed.
- Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
- Your doctor may discuss with you the need to be on a diet. Follow your agreed diet plan carefully.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How long to take it
Take CADUET every day and continue taking it for as long as your doctor tells you.
CADUET helps to lower cholesterol levels and blood pressure and control the symptoms of angina but it does not cure your condition.
It is important to keep taking your medicine even if you feel well. If you stop taking CADUET, your blood pressure and cholesterol levels may rise again.
If you forget to take CADUET
CADUET should be used regularly at the same time each day. If you miss your dose at the usual time:
- if it is less than 12 hours before your next dose, skip the dose you missed and take your next dose when you are meant to.
- otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your tablets, ask your pharmacist for some hints.
If you take too much CADUET
If you think that you have used too much CADUET, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
If you take too many tablets, you may feel dizzy, lightheaded or faint and have an irregular heartbeat.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using CADUET?
Things you should do
Keep all of your doctor's appointments so that your progress can be checked.
Follow the directions from your doctor about regular testing.
Your cholesterol and triglyceride levels and your liver function tests need to be checked regularly while you are taking this medicine.
A regular blood test to check an enzyme called creatine kinase (CK) may be needed. CK in your blood can rise after muscle injury which can be caused by medicines used to treat cholesterol or triglycerides, such as CADUET.
This helps to make sure that CADUET is working and to avoid some possible side-effects.
Your blood pressure may also be checked regularly.
If you are about to start any new medicine, tell your doctor or pharmacist that you are taking CADUET.
If you become pregnant while taking CADUET, stop taking it and tell your doctor immediately.
Remind any doctor, dentist or pharmacist you visit that you are using CADUET.
Things that would be helpful for your condition:
Some self-help measures suggested below may assist your condition. Your doctor or pharmacist can give you more information about these measures.
- weight: While you are taking CADUET, you may need to follow a diet plan agreed to with your doctor. This may include measures to lose some weight.
- exercise: Regular exercise can help lower your cholesterol levels and blood pressure and can strengthen your heart. It is important not to overdo it. Before commencing regular exercise you should consult your doctor who will suggest the most suitable exercise for you. If you experience any discomfort when exercising, see your doctor.
- avoid smoking: Smoking increases the risk of you suffering from heart problems. Your doctor may advise you to stop smoking.
- reduce salt: Your doctor may advise you to watch the amount of salt in your diet. To reduce your salt intake, avoid using salt at the table or in cooking.
Things you should not do
- Do not take CADUET to treat any other conditions unless your doctor tells you to.
- Do not give this medicine to anyone else, even if they have the same condition as you.
Avoid eating large quantities of grapefruit or drinking large quantities of grapefruit juice.
Grapefruit juice contains one or more components that alter the metabolism of some medicines, including CADUET.
Drinking very large quantities (over 1.2 litres) of grapefruit juice each day while taking CADUET increases your chance of getting side effects.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how CADUET affects you.
CADUET may cause dizziness or drowsiness in some people and affect alertness.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Avoid drinking large quantities of alcohol. Tell your doctor if you drink alcohol.
Drinking large quantities of alcohol while taking CADUET may increase your chance of getting liver problems. Excessive alcohol intake can raise your cholesterol levels or affect your liver function, which could increase the chance of you getting unwanted side effects.
Your doctor may discuss with you whether you should reduce the amount of alcohol you drink.
Looking after your medicine
- Keep your tablets in the pack until it is time to take them. If you take your tablets out of the pack they may not keep well.
- Keep your tablets in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If your doctor tells you to stop taking CADUET or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. All side effects should be reported to a health professional. |
Serious side effects
| Serious side effects | What to do |
These are serious side effects that may require medical attention:
| Tell your doctor as soon as possible if you notice any of these side effects. |
The below list includes very serious side effects. You may need urgent medical attention or hospitalisation:
| Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What CADUET contains
| Active ingredients (main ingredients) | Caduet tablets contain the following strength combinations of the active ingredients amlodipine/atorvastatin:
|
| Other ingredients (inactive ingredients) |
|
Do not take this medicine if you are allergic to any of these ingredients.
CADUET does not contain gluten, sugar or lactose.
What CADUET looks like
CADUET Tablets are available in eight strengths:
- Caduet 5 mg/10 mg - white, oval, film coated tablets debossed with "AT10" on one side and "A5" on the other
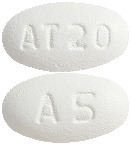
(AUST R 100695) - Caduet 5 mg/20 mg - white, oval, film coated tablets debossed with "AT20" on one side and "A5" on the other
(AUST R 100696) - Caduet 5 mg/40 mg - white, oval, film coated tablets debossed with "AT40" on one side and "A5" on the other
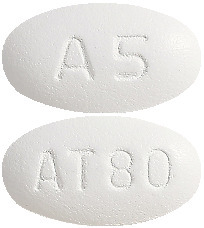
(AUST R 100697) - Caduet 5 mg/80 mg - white, oval, film coated tablets debossed with "AT80" on one side and "A5" on the other
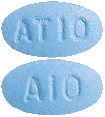
(AUST R 100698) - Caduet 10 mg/10 mg - blue, oval, film coated tablets debossed with "AT10" on one side and "A10" on the other
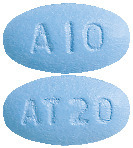
(AUST R 100700) - Caduet 10 mg/20 mg - blue, oval, film coated tablets debossed with "AT20" on one side "A10" on the other (AUST R 100701)
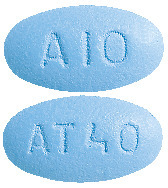
- Caduet 10 mg/40 mg - blue, oval, film coated tablets debossed with "AT40" on one side and "A10" on the other
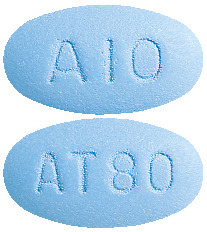
(AUST R 100702) - Caduet 10 mg/80 mg - blue, oval, film coated tablets debossed with "AT80" on one side and "A10" on the other
(AUST R 100703)
A box contains 10 or 30 tablets.
Who distributes CADUET
Aspen Pharmacare Australia Pty Ltd
34-36 Chandos Street
St Leonards NSW 2065
Australia
www.aspenpharma.com.au
This leaflet was prepared in October 2023.
Published by MIMS January 2024



 The safety profile of the combination product is consistent with the adverse events previously reported for amlodipine and/or atorvastatin that are detailed below.
The safety profile of the combination product is consistent with the adverse events previously reported for amlodipine and/or atorvastatin that are detailed below. Other adverse events which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following (see Table 3).
Other adverse events which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following (see Table 3). The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship.
The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship. In the AVALON double blind, placebo controlled study, a total of 847 patients with comorbid hypertension and dyslipidaemia received once daily placebo, 5 mg amlodipine, 10 mg of atorvastatin or the combination of 5 mg amlodipine and 10 mg atorvastatin. The primary objective of the study was the percentage of patients on the combination of amlodipine and atorvastatin reaching JNC VI and NCEP III goals compared to atorvastatin, amlodipine and placebo alone. The results following 8 weeks of treatment are summarised in Table 5. Significantly more patients treated with the combination (45.5%) reached both their blood pressure (BP) and LDL-C goals compared to amlodipine or atorvastatin alone. Caduet was not studied in patients with decompensated chronic cardiac failure or post-myocardial infarction (within 3 to 6 months).
In the AVALON double blind, placebo controlled study, a total of 847 patients with comorbid hypertension and dyslipidaemia received once daily placebo, 5 mg amlodipine, 10 mg of atorvastatin or the combination of 5 mg amlodipine and 10 mg atorvastatin. The primary objective of the study was the percentage of patients on the combination of amlodipine and atorvastatin reaching JNC VI and NCEP III goals compared to atorvastatin, amlodipine and placebo alone. The results following 8 weeks of treatment are summarised in Table 5. Significantly more patients treated with the combination (45.5%) reached both their blood pressure (BP) and LDL-C goals compared to amlodipine or atorvastatin alone. Caduet was not studied in patients with decompensated chronic cardiac failure or post-myocardial infarction (within 3 to 6 months).
 In a long-term (follow-up at least 6 months, mean 13.8 months) placebo controlled mortality/ morbidity study of amlodipine 5 mg to 10 mg in 1153 patients with NYHA classes III (n = 931) or IV (n = 222) heart failure on stable doses of diuretics, digoxin and angiotensin converting enzyme (ACE) inhibitors, amlodipine had no effect on the primary endpoint of the study which was the combined endpoint of all cause mortality and cardiac morbidity (as defined by life threatening arrhythmia, acute myocardial infarction or hospitalisation for worsened heart failure) or on NYHA classification or symptoms of heart failure. Total combined all cause mortality and cardiac morbidity events were 222/571 (39%) for patients on amlodipine and 246/583 (42%) for patients on placebo: the cardiac morbid events represented about 25% of the endpoints in the study.
In a long-term (follow-up at least 6 months, mean 13.8 months) placebo controlled mortality/ morbidity study of amlodipine 5 mg to 10 mg in 1153 patients with NYHA classes III (n = 931) or IV (n = 222) heart failure on stable doses of diuretics, digoxin and angiotensin converting enzyme (ACE) inhibitors, amlodipine had no effect on the primary endpoint of the study which was the combined endpoint of all cause mortality and cardiac morbidity (as defined by life threatening arrhythmia, acute myocardial infarction or hospitalisation for worsened heart failure) or on NYHA classification or symptoms of heart failure. Total combined all cause mortality and cardiac morbidity events were 222/571 (39%) for patients on amlodipine and 246/583 (42%) for patients on placebo: the cardiac morbid events represented about 25% of the endpoints in the study. In three further trials, 1,148 patients with either heterozygous familial hypercholesterolaemia, nonfamilial forms of hypercholesterolaemia or mixed dyslipidaemia were treated with atorvastatin for one year. The results were consistent with those of the dose response study and were maintained for the duration of therapy.
In three further trials, 1,148 patients with either heterozygous familial hypercholesterolaemia, nonfamilial forms of hypercholesterolaemia or mixed dyslipidaemia were treated with atorvastatin for one year. The results were consistent with those of the dose response study and were maintained for the duration of therapy. The primary endpoint examined in ASCOT was the rate of fatal coronary heart disease or nonfatal myocardial infarction over 3.3 years. These coronary events occurred in 1.9% of atorvastatin treated patients compared to 3% of placebo treated patients, a relative risk reduction of 36% (p = 0.0005) (Table 8). Although this difference was statistically significant for the whole trial population, this difference was not statistically significant in specified subgroups such as diabetes, patients with left ventricular hypertrophy (LVH), previous vascular disease or metabolic syndrome.
The primary endpoint examined in ASCOT was the rate of fatal coronary heart disease or nonfatal myocardial infarction over 3.3 years. These coronary events occurred in 1.9% of atorvastatin treated patients compared to 3% of placebo treated patients, a relative risk reduction of 36% (p = 0.0005) (Table 8). Although this difference was statistically significant for the whole trial population, this difference was not statistically significant in specified subgroups such as diabetes, patients with left ventricular hypertrophy (LVH), previous vascular disease or metabolic syndrome.

 Chemical name: 3-ethyl, 5-methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)-6-methyl-1, 4-dihydropyridine-3, 5-dicarboxylate benzene sulphonate.
Chemical name: 3-ethyl, 5-methyl (4RS)-2-[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)-6-methyl-1, 4-dihydropyridine-3, 5-dicarboxylate benzene sulphonate. Chemical name: [R-(R*,R*)]-2-(4-fluorophenyl)-β,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1).
Chemical name: [R-(R*,R*)]-2-(4-fluorophenyl)-β,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1).