SUMMARY CMI
CELAPRAM®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using CELAPRAM?
CELAPRAM contains the active ingredient citalopram hydrobromide. CELAPRAM is used to treat depression. For more information, see Section 1. Why am I using CELAPRAM? in the full CMI.
2. What should I know before I use CELAPRAM?
Do not use if you have ever had an allergic reaction to citalopram or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use CELAPRAM? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with CELAPRAM and affect how it works. Drugs that are known to affect the way the heart beats (for example some heart medicines, antibiotics, asthma medicines, antihistamines) should be avoided while taking CELAPRAM. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use CELAPRAM?
- The dose varies from person to person.
- The standard dose for adults for this medicine is between 20 mg and 40 mg (one to two tablets) per day.
More instructions can be found in Section 4. How do I use CELAPRAM? in the full CMI.
5. What should I know while using CELAPRAM?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines | Be careful before you drive or use any machines or tools until you know how CELAPRAM affects you.
|
| Drinking alcohol | Tell your doctor if you drink alcohol.
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using CELAPRAM? in the full CMI.
6. Are there any side effects?
Tell your doctor if you notice any of the following and they worry you: decreased appetite or loss of appetite, dry mouth diarrhoea, nausea, sleeplessness, fatigue, sleepiness or drowsiness, yawning, increased sweating, sexual disturbances. Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital, if you notice any of the following: thoughts of harming yourself or thoughts of suicide, serious allergic reaction, high fever, agitation, confusion, trembling and abrupt contractions of muscles, mania, hallucinations, seizures, tremors, movement disorders, fast, irregular heartbeat. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
CELAPRAM®
Active ingredient(s): citalopram hydrobromide
Consumer Medicine Information (CMI)
This leaflet provides important information about using CELAPRAM. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using CELAPRAM.
Where to find information in this leaflet:
1. Why am I using CELAPRAM?
2. What should I know before I use CELAPRAM?
3. What if I am taking other medicines?
4. How do I use CELAPRAM?
5. What should I know while using CELAPRAM?
6. Are there any side effects?
7. Product details
1. Why am I using CELAPRAM?
CELAPRAM contains the active ingredient citalopram hydrobromide. CELAPRAM belongs to a group of medicines called selective serotonin reuptake inhibitors (SSRIs). They are thought to work by acting on chemicals in your brain called amines. These amines are involved in controlling mood.
CELAPRAM is used to treat depression.
Depression is longer lasting or more severe than the "low moods" everyone has from time to time due to the stress of everyday life. It is thought to be caused by a chemical imbalance in parts of the brain. This imbalance affects your whole body and can cause emotional and physical symptoms such as feeling low in spirit, loss of interest in activities, being unable to enjoy life, poor appetite or overeating, disturbed sleep, often waking up early, loss of sex drive, lack of energy and feeling guilty over nothing.
CELAPRAM corrects this chemical imbalance and may help relieve the symptoms of depression.
Ask your doctor if you have any questions about why CELAPRAM has been prescribed for you.
Your doctor may have prescribed CELAPRAM for another reason.
2. What should I know before I use CELAPRAM?
Warnings
Do not use CELAPRAM if:
- you have a condition called 'congenital long QT syndrome. At high doses, CELAPRAM can cause changes in the way that your heart beats. See your doctor immediately if you experience an irregular heartbeat, shortness of breath, dizziness or fainting while taking CELAPRAM.
- you are allergic to citalopram hydrobromide, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- the packaging shows signs of tampering or the tablets do not look quite right.
Check with your doctor if you:
- take any medicines for any other condition
- you have allergies to any other substances such as foods, preservatives, or dye. Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing, swelling of the face, lips, tongue or other parts of the body, or rash, itching or hives on the skin.
- Are lactose intolerant, as CELAPRAM tablets contain lactose.
If you have, or have had any of the following medical conditions, tell your doctor before starting CELAPRAM
- Congenital long QT syndrome or other heart conditions. Your doctor may occasionally need to check your heart beat and rhythm with an ECG test
- illnesses which require you to have regular blood test
- A tendency to bleed or bruise easily
- bipolar disorder (manic depression)
- diabetes
- a history of seizures or fits
- liver disease
- kidney disease
- restlessness and/or a need to move often
- raised intraocular pressure (fluid pressure in the eye), or if you are a risk of angle-closure glaucoma.
- epilepsy
- you are receiving electroconvulsive therapy.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Citalopram has been shown to reduce the quality of sperm in animal studies, which theoretically could affect fertility. If you are intending to start a family, ask your doctor for advice.
Do not take CELAPRAM if you are pregnant unless you and your doctor have discussed the risks and benefits involved.
Make sure your doctor and/or midwife know you are on CELAPRAM.
When taken during pregnancy, particularly in the last three months of pregnancy, medicines like CELAPRAM may affect the general condition of your newborn baby and may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your doctor and/or midwife immediately.
If you take CELAPRAM near the end of your pregnancy there may be an increased risk of heavy vaginal bleeding shortly after birth, especially if you have a history of bleeding disorders. Your doctor or midwife should be aware that you are taking CELAPRAM so they can advise you.
If used during pregnancy CIPRAMIL should never be stopped abruptly.
Ask your doctor or pharmacist for advice before taking any medicine.
Do not take CELAPRAM if you are breast-feeding unless you and your doctor have discussed the risks and benefits involved.
It is not recommended that you breast-feed while taking CELAPRAM as it is excreted in breast milk.
CELAPRAM use in children and adolescents
Do not give CELAPRAM to a child or adolescent.
There is no experience with its use in children or adolescents under 18 years old.
CELAPRAM use in the elderly
CELAPRAM can be given to elderly patients over 65 years of age with a reduced dose.
The effects of CELAPRAM in elderly patients are similar to that in other patients.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Do not take CIPRAMIL at the same time as the following other medicines:
- pimozide, a medicine used to treat mental disorders
- monoamine oxidase inhibitors (MAOIs), which are also used for the treatment of depression.
- monoamine oxidase inhibitors such as selegiline which is used in the treatment of Parkinson's Disease
- Do not take CELAPRAM when you are taking a MAOI or when you have been taking a MAOI within the last 14 days.
- Taking CELAPRAM with MAOIs may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions. Your doctor will know when it is safe to start CELAPRAM after the MAOI has been stopped. - the antibiotic linezolid
- Do not take CELAPRAM when you are taking the antibiotic linezolid, or have recently stopped taking linezolid in the last 14-days.
Some medicines may interfere with CELAPRAM and affect how it works. These include:
- monoamine oxidase inhibitors (MAOIs), medicines used to treat depression. Do not take CELAPRAM with MAOIs. CELAPRAM can only be started after you have stopped taking:
- tranylcypromine and phenelzine for at least 14 days
- moclobemide for at least 1 day - other antidepressants, including other SSRIs and tricyclic antidepressants such as imipramine, despiramine
- medicines used to treat mental illnesses such as schizophrenia, depression and mood swings, including antipsychotics and lithium
- linezolid, an antibiotic
- Non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, which are used to treat both pain and inflammation
- sumatriptan, a medicine used to relieve migraines
- tryptophan, an amino acid found in sports and dietary supplements
- ketoconazole, itraconazole, fluconazole, medicines taken to treat fungal infections
- macrolide antibiotics such as erythromycin and clarithromycin
- warfarin, a medicine used to prevent blood clots
- carbamazepine, a medicine used to treat convulsions
- cimetidine and omeprazole, medicines used to treat reflux and stomach ulcers
- St John's Wort (Hypericum perforatum), a herbal remedy used for depression
- tramadol, a medicine used for pain relief
- selegiline, a medicine used to treat Parkinson's disease
- beta-blockers, medicines used to treat high blood pressure and other heart problems such as metoprolol
- antiarrhythmics, medicines used to treat an irregular heart beat
- digoxin, a medicine used to treat heart failure or to control a fast irregular heart beat
- any other medicines for depression, anxiety, obsessive-compulsive disorder or pre-menstrual dysphoric disorder.
These medicines may be affected by CELAPRAM or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
Some combinations of medicines may increase the risk of serious side effects and are potentially life threatening.
Drugs that are known to affect the way the heart beats (for example some heart medicines, antibiotics, asthma medicines, antihistamines) should be avoided while taking CELAPRAM. If it is necessary for you to be on these medicines at the same time as CELAPRAM, your doctor may perform an ECG test to check your heart rate and rhythm.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking CELAPRAM.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect CELAPRAM.
4. How do I use CELAPRAM?
How much to take
- The dose varies from person to person.
- The standard dose for adults for this medicine is between 20 mg and 40 mg (one to two tablets) per day.
- Your doctor may gradually increase this dose depending on how you respond to this medicine.
- The recommended starting dose in elderly patients is 10 mg (half a tablet) per day but may be increased to a maximum of 20 mg (one tablet) per day by your doctor if needed.
- If you have liver problems, or are taking medicines such as cimetidine or omeprazole, the recommended starting dose is 10mg (half a tablet) per day. The dose can be increased to a maximum of 20mg (one tablet) per day.
- Your doctor may have prescribed a different dose. If you have been prescribed or are currently taking doses of CELAPRAM greater than 40mg, talk to your doctor about reducing the dose.
- Follow all directions given to you by your doctor and pharmacist carefully.
- They may differ from the information contained in this leaflet.
- If you do not understand the instructions on the pack or bottle, ask your doctor or pharmacist for help.
When to take CELAPRAM
- Take CELAPRAM at about the same time each day.
Taking it at the same time each day will have the best effect. It will also help you to remember when to take it. - CELAPRAM can be taken with or without food, either in the morning or evening.
How to take CELAPRAM
- Swallow the tablets as a single daily dose with a glass of water.
CELAPRAM 20 mg and 40 mg tablets can be divided in half if advised by your doctor or pharmacist.
How long to take it
- Continue to take CELAPRAM even if it takes some time before you feel any improvement in your condition.
Most medicines of this type take time to work, so do not be discouraged if you do not feel better right away. The treatment of depression may take at least six months. - Continue taking your medicine for as long as your doctor tells you, even if you begin to feel better.
- The underlying illness may persist for a long time and if you stop your treatment too soon, your symptoms may return.
- Do not stop taking this medicine suddenly.
- If CELAPRAM is stopped suddenly you may experience mild, but usually temporary, symptoms such as dizziness, pins and needles, sleep disturbances (vivid dreams, inability to sleep), feeling anxious or agitated, headaches, feeling sick (nausea), vomiting, sweating, tremor (shaking), feeling confused, feeling emotional or irritable, diarrhoea, visual disturbances, or fast or irregular heartbeats.
- When you have completed your course of treatment, the dose of CELAPRAM is gradually reduced over a couple of weeks rather than stopped abruptly.
- Your doctor will tell you how to reduce the dosage so that you do not get these unwanted effects.
If you forget to use CELAPRAM
CELAPRAM should be used regularly at the same time each day. If you miss your dose at the usual time, and remember in less than 12 hours, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do or you have any questions about this, ask your doctor or pharmacist.
If you use too much CELAPRAM
If you think that you have used too much CELAPRAM, you may need urgent medical attention.
Symptoms of an overdose may include nausea (feeling sick), vomiting, dizziness, fast or slow heart beat or changes in heart rhythm, decreased or increased blood pressure, tremor (shaking), agitation, dilated pupils of the eyes, drowsiness, sleepiness, lethargy, sweating, blueish discolouration of the skin and an increase in rate of breathing. Convulsions or coma may occur. A condition called serotonin syndrome may occur with high fever, agitation, confusion, trembling and abrupt contractions of muscles.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using CELAPRAM?
Things you should do
Before starting any new medicine, tell your doctor or pharmacist that you are taking CELAPRAM.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking CELAPRAM.
If you become pregnant while taking CELAPRAM, tell your doctor immediately.
Persons taking CELAPRAM may be more likely to think about killing themselves or actually trying to do so, especially when CELAPRAM is first started or the dose is changed. Tell your doctor immediately if you have thoughts about killing yourself or if you are close to or care for someone taking CELAPRAM who talks about or shows signs of killing him or herself.
Occasionally, the symptoms of depression may include thoughts of suicide or self-harm. It is possible that these symptoms continue or get worse until the full antidepressant effect of the medicine becomes apparent. This is more likely to occur in children, adolescents and young adults under 25 years of age, and you have not used antidepressant medicines before.
Contact your doctor or a mental health professional right away, or go to the nearest hospital for treatment if you or someone you know is showing any of the following warning signs of suicide:
- worsening of your depression
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of self-harm
- increase in aggressive behaviour, irritability, agitation or any other unusual changes in behaviour or mood.
All mentions of suicide or violence must be taken seriously.
Family and carers of people taking CELAPRAM also need to monitor for the above symptoms.
Do not stop taking this medicine or change the dose without consulting your doctor, even if you experience increased anxiety at the beginning of treatment.
At the beginning of treatment, some patients may experience increased anxiety which will disappear during continued treatment.
Visit your doctor regularly so they can check on your progress.
If you plan to have surgery, including dental surgery, tell your doctor or dentist that you are taking CELAPRAM
Call your doctor straight away if you:
- become pregnant while taking CELAPRAM. Do not stop taking your tablets until you have spoken to your doctor.
- experience symptoms such as restlessness or difficulty in sitting or standing still.
These symptoms can occur during the first weeks of treatment. - experience episodes of mania. Some patients with bipolar disorder (manic depression) may enter into a manic phase. This is characterised by profuse and rapidly changing ideas, exaggerated gaiety and excessive physical activity.
Things you should not do
Do not use CELAPRAM to treat any other conditions unless your doctor tells you to.
Do not give CELAPRAM to anyone else, even if they have the same condition as you.
Do not suddenly stop taking CELAPRAM, or change the dose, without checking with your doctor.
Do not let yourself run out of CELAPRAM over weekends or holidays.
If you stop CELAPRAM suddenly, you may get unwanted side effects, such as dizziness, headache and nausea.
Your doctor will tell you how to gradually reduce the amount of CELAPRAM you are taking before stopping completely. This is usually done slowly over one to two weeks.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how CELAPRAM affects you.
CELAPRAM may cause dizziness, visual disturbances or drowsiness in some people. If you experience any of these, do not drive, operate machinery or do anything else that could be dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol.
Avoid alcohol while you are taking this medicine.
It is not advisable to drink alcohol while you are being treated for depression.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Keep your tablets in the pack or bottle until it is time to take them. If you take the tablets out of the pack or bottle they may not keep well.
Store it in a cool dry place where the temperature stays below 25°C, away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
CELAPRAM helps most people with depression, but it may have unwanted side effects in some people.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking CELAPRAM.
Do not be alarmed by this list of possible side effects.
You may not experience any of them.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
These are the more common yet mild side effects of CELAPRAM. Some of these may occur within the first two weeks of treatment and disappear after a short period of time. | Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
These side effects listed above are serious and may need urgent medical attention. | Tell your doctor as soon as possible if you notice any of the following |
| Serious side effects | What to do |
The below are very serious side effects.
The above list includes very serious side effects, which are uncommon or rare. You may need urgent medical attention or hospitalisation. | Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What CELAPRAM contains
| Active ingredient (main ingredient) | Citalopram hydrobromide. CELAPRAM tablets contain 10 mg, 20 mg or 40 mg of citalopram (as citalopram hydrobromide). |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | CELAPRAM contains sugars as lactose and trace amounts of sulfites. |
Do not take this medicine if you are allergic to any of these ingredients.
What CELAPRAM looks like
CELAPRAM 10 mg - round, white, film-coated tablet marked "CM" over "10" on one side and "G" on the other. Each pack contains 28 tablets. (AUST R 93542)
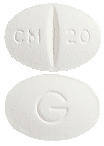
CELAPRAM 20 mg - oval, white, scored film-coated tablet marked "CM breakline 20" on one side and "G" on the other. Each pack and bottle contains 28 tablets. (AUST R 82904, 82905)
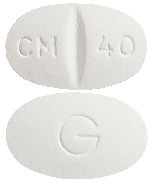
CELAPRAM 40 mg - oval, white, scored, film-coated tablet marked "CM breakline 40" on one side and "G" on the other. Each pack contains 28 tablets. (AUST R 93543)
Who distributes CELAPRAM
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in June 2023.
CELAPRAM® is a Viatris company trade mark
CELAPRAM_cmi\Jun23/00
Published by MIMS July 2023



 Molecular formula: C20H21FN2O, HBr. Molecular weight: 405.3.
Molecular formula: C20H21FN2O, HBr. Molecular weight: 405.3.