What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some common questions about venlafaxine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
You can also download the most up to date leaflet from www.apotex.com.au.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is Chemmart Venlafaxine XR Capsules. It contains the active ingredient venlafaxine (as venlafaxine hydrochloride).
It is used to treat major depression, and to prevent it coming back. It is also used in the treatment of panic attacks and anxiety, including avoidance or fear of social situations.
How it works
Depression can affect your whole body and may cause emotional and physical symptoms such as feeling low in spirit, being unable to enjoy life, poor appetite or overeating, disturbed sleep, loss of sex drive, lack of energy and feeling guilty over nothing.
Excessive anxiety is a condition in which you feel constantly and uncontrollably worried and distressed. It may also make you feel irritable, and cause difficulty in thinking and sleeping. Other common symptoms associated with anxiety may include a dry mouth, a lump in the throat, cold clammy hands, diarrhoea and nausea.
Depression and anxiety are treatable illnesses. Anxiety or tension associated with the normal stress of everyday life usually does not require treatment with medicines.
Venlafaxine belongs to a class of medications for depression, called Serotonin-Noradrenaline Reuptake Inhibitors (SNRIs).
Serotonin and noradrenaline are chemical messengers that allow certain nerves in the brain to work.
Venlafaxine increases the level of these two messengers. Experts think this is how it helps to restore your feeling of wellness.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed Venlafaxine XR for another reason.
This medicine is available only with a doctor's prescription.
There is no evidence that this medicine is addictive.
Use in children
Do not give venlafaxine to children or adolescents under 18 years of age.
The safety and effectiveness of venlafaxine in this age group have not been established.
Before you take this medicine
When you must not take it
Do not take this medicine if you are taking other medicines called Monoamine Oxidase Inhibitors (MAOIs). These may be used for the treatment of depression (phenelzine, tranylcypromine, moclobemide), Parkinson's disease (selegiline) or infections (linezolid) or diagnosis of certain conditions/treatment of certain blood disorders (methylene blue).
There may be others so check with your doctor or pharmacist.
Do not take venlafaxine until 14 days after stopping most MAOIs, and do not take any MAOIs until more than one week after stopping venlafaxine (see also point 7 in "Before You Start to Take it").
(However you may take venlafaxine 24 hours after stopping the reversible MAOI called moclobemide.)
Taking venlafaxine with a MAOI may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
Do not take this medicine if:
- The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
- You have had an allergic reaction to venlafaxine or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body, rash, itching or hives on the skin; fainting or hayfever-like symptoms.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- epilepsy or fits (seizures or convulsions)
- a personal or family history of bipolar disorder
- aggression
- drug abuse or misuse
- blood pressure problems
- diabetes
- acute angle glaucoma or increased pressure in the eye
- a tendency to bleed more than normal
- other blood disorders
- raised cholesterol levels
- problems with your kidneys or liver
- low sodium levels in your blood, or a condition called SIADH (Syndrome of Inappropriate Antidiuretic Hormone Secretion)
- thoughts or actions relating to self-harm or suicide
- problems with your heart
Your doctor may wish to do some heart tests such as an electrocardiogram (ECG) or blood tests during treatment with venlafaxine. - any other medical conditions.
- You are currently pregnant or you plan to become pregnant. There have been reports that babies exposed to venlafaxine and other antidepressants during the third trimester of pregnancy may develop complications after birth.
Do not take this medicine whilst pregnant until you and your doctor have discussed the risks and benefits involved. - You are currently breastfeeding or you plan to breastfeed.
Venlafaxine passes into breast milk and there is a possibility that the breastfed baby may be affected. For this reason, the use of venlafaxine is not recommended if you are breastfeeding. Do not take this medicine whilst breastfeeding until you and your doctor have discussed the risks and benefits involved. - You are planning to have surgery or any medical tests or treatments.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Some medicines may interact with venlafaxine. These include:
- Medicines called Monoamine Oxidase Inhibitors (MAOIs). MAOIs may be used for the treatment of depression (phenelzine, tranylcypromine, moclobemide), Parkinson's disease (selegiline), infections (linezolid) and diagnosing certain conditions/treating certain blood disorders (methylene blue). Do not take venlafaxine until 14 days after stopping most MAOIs, and do not take any MAOIs until more than one week after stopping venlafaxine. For moclobemide, do not take venlafaxine until at least 24 hours after stopping moclobemide, and do not take moclobemide until more than one week after stopping venlafaxine.
- St John's wort (Hypericum perforatum) or tryptophan, contained in some multivitamin and herbal preparations, which can be bought without a prescription
- Medicines used to treat Parkinson's disease, called dopamine agonists
- Any other medications for depression, anxiety, obsessive-compulsive disorder or premenstrual dysphoric disorder
- Haloperidol, risperidone, lithium or clozapine (used to treat conditions which affect the way you think, feel or act)
- Tramadol (a strong pain killer)
- Any other medicine which may have an effect on the brain
- Cimetidine (used to treat reflux and stomach ulcers)
- Medicines called triptans (used to treat migraine)
- Amiodarone or quinidine (used to treat irregular heartbeats)
- Medicines used to prevent blood clots - e.g. warfarin and clopidogrel
- Medicines called diuretics, used for reducing water in your body and treating high blood pressure
- Medicines used to treat diabetes.
If you are taking any of these you may need a different dose or you may need to take different medicines.
Your doctor may also wish to do some heart tests such as an electrocardiogram (ECG) or blood tests if you are using any of the following whilst taking venlafaxine:
- Warfarin (used to prevent blood clotting)
- Indinavir (an antiviral)
- Erythromycin (used to treat infections)
- Ketoconazole or fluconazole (used as antifungal medicines)
- Medications for weight loss, including phentermine and sibutramine
- Metoprolol (used to treat high blood pressure or angina)
- Any medications that can affect your heart beat
- Grapefruit juice.
Other interactions not listed above may also occur.
How to take this medicine
Follow carefully all directions given to you by your doctor or pharmacist. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor or pharmacist will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
The usual starting dose is 75 mg taken once daily.
If necessary, after two weeks, your doctor may increase your dose. The maximum you should take in one day is 225 mg.
The dose may be much lower if you have liver or kidney problems.
If you have kidney or liver problems, you may need a lower dose of venlafaxine.
If you have heart problems and your doctor wishes to increase your dose of venlafaxine, your doctor may first do some blood tests or heart tests such as an electrocardiogram (ECG).
Do not stop taking your medicine or change your dosage without first checking with your doctor.
How to take it
Swallow the capsules whole with a glass of water or other non-alcoholic liquid. Do not divide, crush, chew or place the capsules in water.
Inside Venlafaxine XR capsules are tablets that contain the venlafaxine active ingredient. As the medicine travels the length of your gastrointestinal tract, venlafaxine is slowly released.
When to take it
Take venlafaxine once daily with food, either in the morning or in the evening.
Take this medicine at the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
Avoid drinking alcohol while you are taking venlafaxine.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
Although you may begin to feel better after two weeks, it may take several weeks before you feel much better. It is important to give venlafaxine time to work.
Even when you feel well again, you may need to keep taking venlafaxine for several months to make sure the benefits last. Discuss this with your doctor and do not stop taking venlafaxine until gaining your doctor's agreement.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is less than 12 hours until your next dose, skip the missed dose and take your next dose at the usual time. Otherwise take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses.
This may increase the chance of you experiencing side effects.
Contact your doctor if you have missed more than two doses in a row.
Always finish the capsules you are taking in the current pack before you start a new pack.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency Department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much venlafaxine, you may:
- Feel sleepy
- Vomit
- Have an increased heart rate or changes in heart rhythm
- Have a seizure (fits)
- Have breathing difficulties
- Become unconscious
- Have dilated pupils.
While you are taking this medicine
Things you must do
Watch carefully for signs that your depression is getting worse, especially in the first few weeks of treatment or if your dose has changed.
Tell your doctor immediately if you experience any of the following symptoms, especially if they are severe, you have not had these symptoms before or they happen very suddenly:
- anxiety or agitation
- panic attacks
- difficulty sleeping
- irritability
- aggressiveness
- hostility or impulsiveness
- restlessness
- overactivity or uninhibited behaviour
- other unusual changes in behaviour
- thoughts of suicide.
Tell your doctor immediately if you have any thoughts about suicide or doing harm to yourself.
Warning signs of suicide:
All thoughts or talk about suicide or violence are serious. If you or someone you know is showing the following warning signs, either contact your doctor or a mental health advisor right away or go to the nearest hospital for treatment.
- thoughts or talk about death or suicide.
- thoughts or talk about self-harm or doing harm to others.
- any recent attempts of self-harm.
- an increase in aggressive behaviour, irritability or agitation.
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you become pregnant.
Make sure your midwife and/or doctor know immediately that you are pregnant and taking venlafaxine, as there is a possibility of problems developing in unborn children. Symptoms such as feeding difficulty, vomiting, tremor, irritability and constant crying have been reported rarely in newborn babies after mothers have taken venlafaxine in the last 3 months of pregnancy. In addition, when taken particularly in the last 3 months of pregnancy, medicines like venlafaxine may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately. - you are breastfeeding or are planning to breastfeed
- you are about to have any blood tests
- you are going to have surgery or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
Some people (especially older people and/or those taking diuretics/water tablets) may experience a lack of sodium in the blood when taking this medicine. Tell your doctor if you get a headache or start to feel dizzy, confused, forgetful, weak, unsteady or unable to concentrate. Make sure to drink plenty of fluids.
If you are being treated for depression, be sure to discuss with your doctor any problems you may have and how you feel, especially any feelings of severe sadness, thoughts of suicide, bursts of unusual energy, anger or aggression, or if you become particularly agitated or restless.
This will help your doctor to determine the best treatment for you.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours
- Take your medicine to treat any other condition unless your doctor or pharmacist tells you to
- Stop taking your medicine, or change the dosage, without first checking with your doctor
- Suddenly stop taking these capsules if you have been taking them for some time.
Check with your doctor for the best way to slowly reduce the amount of venlafaxine you are taking before stopping completely.
Side effects from stopping treatment with venlafaxine may include:
- headache
- nausea and vomiting
- dizziness
- insomnia
- nervousness
- anxiety
- confusion and agitation
- diarrhoea
- sweating
- loss of appetite
- tremor
- flu-like symptoms
- impaired coordination and balance
- tingling or numbness of the hands and feet.
Slowly reducing the amount of venlafaxine being taken reduces the possibility of these effects occurring.
Things to be careful of
Venlafaxine may make you feel drowsy. Be careful when driving or operating machinery until you know how this medicine affects you.
Possible side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Venlafaxine XR or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Tell your doctor or pharmacist if you notice any of the following.
This list includes the more common side effects. Mostly, these are mild:
Stomach or bowel problems such as:
- nausea or vomiting
- loss of appetite
- diarrhoea
- constipation
- wind
Changes in your behaviour such as:
- difficulty sleeping or abnormal dreams
- sexual function problems such as delayed ejaculation, problems with erection, decreased sex drive or difficulties achieving orgasm
- nervousness
- teeth grinding
- a feeling of apathy or not caring about things or not being part of your body
- excessive enthusiasm or desire, delusions (mania).
Difficulty thinking or working because of:
- yawning
- feeling sedated or drowsy
- fainting or dizziness after standing up
- changes in muscle tone
- muscle weakness or fatigue
- restlessness, agitation or difficulty sitting still
- headache
- heavy or irregular menstrual periods.
Changes in your appearance such as:
- sweating (including night sweats)
- hot flushes
- rash
- hair loss
- itchiness
- weight loss or weight gain
- unusual milk production
Changes in your sight, hearing, taste or touch such as:
- dilated pupils
- sensitivity to sunlight
- ringing in the ears
- altered taste
- dry mouth
- sore throat
- numbness or pins and needles.
Tell your doctor as soon as possible if you notice any of the following.
These may be serious side effects. You may need medical attention. Most of these side effects are rare.
- blurred vision
- cloudy urine, problems passing urine, passing urine more frequently, or being unable to control urination
- muscle tremors, spasms, twitching, jerky movements or sustained muscle contractions
- impaired coordination and balance
- increase in bone fractures
- abnormal facial movements such as tongue thrusting, repetitive chewing, jaw swinging, or grimacing
- hallucinations
- confusion
- excessively overactive
- rapid heart beat
- problems with breathing
- bleeding or bruising more easily than normal.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and are usually very rare. You may need urgent medical attention or hospitalisation.
- fits or seizures, which may be accompanied by a sudden fever
- symptoms of sudden fever with sweating, rapid heartbeat and muscle stiffness, which may lead to loss of consciousness
- palpitations, fainting, shortness of breath or chest pain
- dark, red or cola coloured urine, muscle weakness and tenderness, stiffness or aching
- stomach pain, yellowing of the skin, nausea, fever, clammy skin and sweating
- yellowing of the skin or eyeballs, fever, fatigue, loss of appetite, dark coloured urine or light coloured bowel movements
- a severe skin reaction with painful red areas and large blisters, accompanied by fever and chills, aching muscles and generally feeling unwell.
- symptoms of a high fever, agitation, confusion, trembling and abrupt contractions of muscles
- signs of an infection such as severe chills, fever, sore throat and mouth ulcers
- black sticky bowel motions or bloody diarrhoea.
Other changes you may not be aware of which can be identified by blood tests:
- increased blood pressure
- increase in blood cholesterol levels.
Other side effects not listed above may occur in some patients.
Allergic reactions
If you think you are having an allergic reaction to Venlafaxine XR, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- shortness of breath, wheezing or difficulty breathing.
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
- fainting
- hayfever-like symptoms
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What Chemmart Venlafaxine XR looks like
75 mg capsules
The capsules have an opaque flesh-coloured cap and body.
Blister pack of 28 capsules.
150 mg capsules
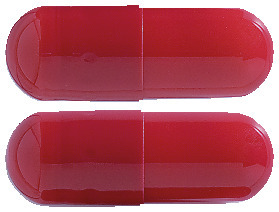
The capsules have an opaque scarlet-coloured cap and body.
Blister pack of 28 capsules.
Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each capsule contains 75 or 150 mg of venlafaxine (as venlafaxine hydrochloride) as the active ingredient.
It also contains the following inactive ingredients:
- Hypromellose
- Sodium lauryl sulfate
- Magnesium stearate
- Ammonio methacrylate copolymer
- Basic butylated methacrylate copolymer
- Titanium dioxide (E171, CI 77891)
- Gelatin
- Red iron oxide (E172, C177491)
This medicine is gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
Chemmart Venlafaxine XR 75 mg capsules (blisters)
AUST R: 177458
Chemmart Venlafaxine XR 150 mg capsules (blisters)
AUST R: 177459
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park, NSW 2113
This leaflet was last updated in January 2017





 Examination of subsets of the population studied did not reveal any differential responsiveness on the basis of gender. There was insufficient information to determine the effect of age or race on outcome in these studies.
Examination of subsets of the population studied did not reveal any differential responsiveness on the basis of gender. There was insufficient information to determine the effect of age or race on outcome in these studies.
 Below are adverse reactions from combined analyses of the clinical studies for major depression, generalised anxiety disorder, social anxiety disorder, and panic disorder. The adverse reactions have been presented using the CIOMS frequency categories: common: ≥ 1%; uncommon: ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%.
Below are adverse reactions from combined analyses of the clinical studies for major depression, generalised anxiety disorder, social anxiety disorder, and panic disorder. The adverse reactions have been presented using the CIOMS frequency categories: common: ≥ 1%; uncommon: ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%.