What is in this leaflet
This leaflet answers some common questions about Clarithromycin AN tablets.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking CLARITHROMYCIN AN against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What CLARITHROMYCIN AN is used for
CLARITHROMYCIN AN is used to treat certain bacterial infections, including the following:
- respiratory tract infections
- skin infections
- peptic ulcer
CLARITHROMYCIN AN is also used to prevent a specific bacterial infection associated with HIV infection.
Your doctor, however, may have prescribed CLARITHROMYCIN AN for another purpose. Ask your doctor if you have any questions about why CLARITHROMYCIN AN has been prescribed for you.
CLARITHROMYCIN AN is an antibiotic that belongs to the group of medicines called macrolides. These medicines work by killing or stopping the growth of bacteria which cause infections.
CLARITHROMYCIN AN will not work against infections caused by viruses, such as colds or flu.
CLARITHROMYCIN AN is available only with a doctor’s prescription.
CLARITHROMYCIN AN to treat peptic ulcer
Peptic ulcers are associated with an infection in the intestine and stomach by a bacteria called Helicobacter pylori (H. pylori). Nearly all patients with peptic ulcers are infected with this bacteria.
The H. pylori infection can be treated with a combination of CLARITHROMYCIN AN (clarithromycin), another antibiotic (amoxycillin) and another medicine called omeprazole (used to control the acidity of the stomach).
However, the best combination of tablets to treat Helicobacter pylori infection is yet to be determined. Your doctor will determine the best combination for you.
If your symptoms return, consult your doctor. It is possible that CLARITHROMYCIN AN may no longer be effective in killing the Helicobacter pylori infection and a different antibiotic may be needed.
Before You Take CLARITHROMYCIN AN
When you must not take it
Do not take CLARITHROMYCIN AN if:
- You have ever had an allergic reaction to medicines containing clarithromycin or to other antibiotics from the macrolide family. These may include:
- erythromycin (EES, Erythrocin, Eryc, Emycin, EMU-V)
- roxithromycin (Rulide, Biaxsig)
- azithromycin (Zithromax) - You are allergic to any of the ingredients listed at the end of this leaflet.
- If you have an allergic reaction to CLARITHROMYCIN AN you may have some of the following symptoms:
- rash
- itching or hives
- swelling of the face, lips, tongue or other parts of the body
- shortness of breath, wheezing or difficulty in breathing - You have severe liver problems or poor kidney function.
- You have an irregular heart beat.
- The packaging is torn or shows any signs of tampering.
- The use by date (Exp.) printed on the pack has passed.
If it has expired or is damaged return it to your pharmacist for disposal.
Do not take CLARITHROMYCIN AN if you are taking the following medicines:
- astemizole or terfenadine (commonly used to treat allergy symptoms - these medicines may be available without prescription)
- cisapride (used to relieve certain stomach problems)
- pimozide (used to treat schizophrenia)
- ergotamine or dihydroergotamine (used to treat headaches)
- lovastatin or simvastatin (used to treat high cholesterol)
Before you start to take it
Tell your doctor if:
- You are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking CLARITHROMYCIN AN when pregnant.
- You are breastfeeding or wish to breastfeed. Your doctor will discuss the risks and benefits of taking CLARITHROMYCIN AN when breastfeeding.
- You have, or have ever had, any other health problems or medical conditions, including liver problems or poor kidney function.
- You are allergic to any other medicines, foods, dyes or preservatives.
- You have myasthenia gravis, a condition in which the muscles become weak and tire easily.
If you have not told your doctor about any of the above, tell them before you start taking or are given CLARITHROMYCIN AN.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may affect the way CLARITHROMYCIN AN works. These include the following medicines:
- digoxin, quinidine, disopyramide (used to treat heart failure)
- warfarin (used to prevent blood clotting)
- phenytoin, carbamazepine, hexobarbital, sodium valproate (used to treat epilepsy)
- theophylline (used to treat asthma)
- triazolam, midazolam, alprazolam (used to treat sleeplessness and anxiety)
- cilostazol (used to treat poor circulation)
- rosuvastatin, atorvastatin (used to treat high cholesterol)
- methylprednisolone (a corticosteroid)
- vinblastine (used to treat cancer)
- sildenafil, tadalafil, vardenafil (used to treat erectile dysfunction in adult males)
- cyclosporin, tacrolimus (medicines affecting the immune system)
- medicines used to treat HIV infection
- rifabutin, rifampicin (used to treat some infections)
- repaglinide, nateglinide, pioglitazone, and rosiglitazone (used to treat diabetes)
- insulin (used to treat diabetes)
- colchicine (used to treat gout)
- verapamil (used to treat high blood pressure)
- fluoxetine (used to treat depression)
- omeprazole (used to treat stomach problems)
- tolterodine (used to treat bladder problems)
- herbal medicines such as St John’s Wort
These medicines may be affected by CLARITHROMYCIN AN or may affect how well CLARITHROMYCIN AN works. Your doctor or pharmacist can tell you what to do if you are taking any of these medicines. They also have a more complete list of medicines to be careful with or avoid while taking CLARITHROMYCIN AN.
How to take CLARITHROMYCIN AN
Your doctor will tell you how much to take and when to take it. Take CLARITHROMYCIN AN exactly as directed by your doctor.
This may differ from the information contained in this leaflet.
How much to take
The dose of CLARITHROMYCIN AN will depend on the infection to be treated.
For respiratory tract infections and skin infections, the usual adult dose is one CLARITHROMYCIN AN 250 mg tablet twice a day.
For more severe infections, the dose can be increased to two CLARITHROMYCIN AN 250 mg tablet twice a day.
Your doctor will adjust the amount or frequency of your doses according to the infection being treated and the severity of your condition.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How to take it
CLARITHROMYCIN AN tablets should be swallowed whole with a glass of water.
How long to take it
Keep taking CLARITHROMYCIN AN until you finish the pack or for as long as your doctor tells you.
If you are being treated for an infection, CLARITHROMYCIN AN is usually taken for one or two weeks.
Do not stop taking CLARITHROMYCIN AN, even if you feel better after a few days, unless advised by your doctor.
Your infection may not clear completely if you stop taking your medicine too soon.
Check with your doctor if you are not sure how long you should be taking CLARITHROMYCIN AN.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you miss more than one dose, or are not sure what to do, check with your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much CLARITHROMYCIN AN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Keep telephone numbers for these places/services handy.
If you take too much CLARITHROMYCIN AN, you may develop severe gastrointestinal symptoms, liver problems, or allergic reactions.
While you are taking CLARITHROMYCIN AN
Things you must do
If you are taking CLARITHROMYCIN AN for an infection and your symptoms do not improve within a few days, or if they become worse, tell your doctor.
If you become pregnant while taking CLARITHROMYCIN AN, tell your doctor.
If you get severe diarrhoea, tell your doctor or pharmacist immediately. Do this even if it occurs several weeks after stopping CLARITHROMYCIN AN.
Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take any medicine to stop your diarrhoea without first checking with your doctor.
If you have to have any urine tests, tell your doctor you are taking CLARITHROMYCIN AN as it may affect the results of some laboratory tests.
If you are about to start taking a new medicine, tell your doctor or pharmacist that you are taking CLARITHROMYCIN AN.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking CLARITHROMYCIN AN.
Things you must not do
Do not use CLARITHROMYCIN AN to treat any other complaints unless your doctor says so.
Do not give CLARITHROMYCIN AN to anyone else, even if their symptoms seem similar to yours.
Things to be careful of
Be careful driving or operating machinery until you know how CLARITHROMYCIN AN affects you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking CLARITHROMYCIN AN.
CLARITHROMYCIN AN treats infections in most people, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
While you are taking Clarithromycin AN
Tell your doctor if you experience any of the following and they worry you:
- stomach cramps and pains
- nausea, vomiting and severe diarrhoea
- oral thrush or vaginal thrush
- change in taste sensation
- headache
Tell your doctor immediately if you notice any of the following, as you may need urgent medical care:
- yellowing of the eyes or skin (jaundice)
- feeling generally unwell and having poor appetite
- hearing disturbances
- chest pain
- dizziness, confusion, hallucinations, convulsions
- any type of skin rash, itching, hives
- severe diarrhoea, especially if bloody
- severe upper stomach pain, with nausea and vomiting (pancreatitis)
Stop taking CLARITHROMYCIN AN and tell your doctor immediately or go to casualty at your nearest hospital if any of the following happen:
- swelling of the face, lips, mouth, throat or neck which may cause difficulty in swallowing or breathing or sudden collapse
After you have finished taking Clarithromycin AN
Tell your doctor immediately if you notice any of the following side effects, even if they occur several weeks after stopping treatment with CLARITHROMYCIN AN:
- severe stomach or abdominal cramps
- watery and severe diarrhoea, which may also be bloody
- fever, in combination with one or both of the above.
These are rare but serious side effects. You may have a serious condition affecting your bowel and you may need urgent medical care.
Do not take any diarrhoea medicine without first checking with your doctor.
Other side effects not listed above may also occur in some patients. Ask your doctor or pharmacist for more information about side effects, as they have a more complete list of side effects. Inform your doctor promptly about these or any other symptoms. If the condition persists or worsens, seek medical attention. Do not be alarmed by this list of possible side effects. They do not occur often and you are unlikely to experience any of them.
Tell your doctor if you notice anything that is making you feel unwell while you are taking, or soon after you have finished taking CLARITHROMYCIN AN, even if it is not on this list.
After taking CLARITHROMYCIN AN
Storage
Keep your medicine where children cannot reach them.
A locked cupboard at least 1.5 metres above the ground is a good place to store medicines.
Keep the pack away from sources of heat.
Keep CLARITHROMYCIN AN tablets in a cool dry place, protected from light, where the temperature stays below 30°C.
Do not keep CLARITHROMYCIN AN or any other medicine in the bathroom or near a sink. Do not leave CLARITHROMYCIN AN in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking CLARITHROMYCIN AN, or your medicine has passed its expiry date, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
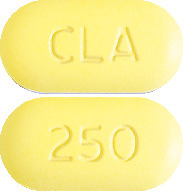
CLARITHROMYCIN AN 250 mg tablets (AUST R 184263) are bright yellow, capsule shaped tablets with ‘CLA’ engraved on one side and ‘250’ engraved on the other side.
Ingredients
Active Ingredient:
The active ingredient in CLARITHROMYCIN AN tablets is clarithromycin. Each tablet contains 250 mg of clarithromycin.
Other Ingredients:
- croscarmellose sodium,
- pregelatinised maize starch,
- microcrystalline cellulose,
- povidone,
- stearic acid,
- magnesium stearate,
- colloidal anhydrous silica,
- quinoline yellow,
- Opadry White Y-1-7000 hypromellose,
- quinoline yellow,
- aluminium lake,
- vanillin flavour.
CLARITHROMYCIN AN tablets do not contain lactose or gluten.
Name and Address of the Sponsor
Scentia Pharmaceuticals Pty Ltd
8 - 12 Ordish Road
Dandenong South,
VIC - 3175
Australia
Date of Preparation
January 2014
Doc ID: 51.AN.M.1.0


 Information was obtained regarding the penetration of clarithromycin in middle ear fluid in paediatric patients with otitis media. Approximately 2.5 hours after receiving the fifth dose (7.5 mg/kg twice daily) the mean concentration of clarithromycin was 2.53 microgram/g fluid in the middle ear, and for the 14-OH metabolite was 1.27 microgram/g. The concentrations of parent drug and 14-OH metabolite were variable, with two-thirds of patients having levels greater than the corresponding concentration in serum and one-third of patients having levels similar or lower. The mean ratio was 2.48 ± 3.57.
Information was obtained regarding the penetration of clarithromycin in middle ear fluid in paediatric patients with otitis media. Approximately 2.5 hours after receiving the fifth dose (7.5 mg/kg twice daily) the mean concentration of clarithromycin was 2.53 microgram/g fluid in the middle ear, and for the 14-OH metabolite was 1.27 microgram/g. The concentrations of parent drug and 14-OH metabolite were variable, with two-thirds of patients having levels greater than the corresponding concentration in serum and one-third of patients having levels similar or lower. The mean ratio was 2.48 ± 3.57.
