What is in this leaflet?
Please read this leaflet carefully before you take Daraprim tablets.
This leaflet answers some common questions about Daraprim tablets. It does not contain all of the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the expected benefits of you taking Daraprim tablets against the risks this medicine could have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What are Daraprim tablets used for?
Daraprim tablets are used:
- to treat toxoplasmosis infections when taken with another antibiotic called a sulphonamide together with a folic acid supplement.
Daraprim belongs to a group of medicines called antiprotozoals, which are used against infections of the blood caused by the parasites called Plasmodium which causes a disease called toxoplasmosis.
Daraprim tablets work by disrupting the way that proteins and genetic material are made inside the parasites.
Your doctor may have prescribed Daraprim tablets for another reason. Please ask your doctor why Daraprim has been prescribed for you.
Daraprim tablets are not addictive.
Before you take Daraprim tablets
Do not take if:
You must not take Daraprim tablets if:
- you have ever had an allergic reaction to pyrimethamine or any of the ingredients listed toward the end of this leaflet. (See “Ingredients”).
- the expiry date (EXP) printed on the pack has passed.
- the packaging is torn or shows signs of tampering.
Tell your doctor if:
You must tell your doctor if:
- you are allergic to foods, dyes, preservatives or any other medicines.
- you have kidney, liver or blood disease (anaemia).
- you have previously been diagnosed as having a low level of folic acid in your blood.
- you are taking or likely to be taking any of the following medicines:
- anti-infectives (e.g. trimethoprim or co-trimoxazole, zidovudine)
- sleeping tablets (e.g. lorazepam)
- anti-cancer medicines (e.g. methotrexate, daunorubicin, cytosine arabinoside)
- blood thinning tablets (e.g. warfarin)
- antimalarial medicines (e.g. quinine, proguanil). - you are taking any other medicines, including medicines you buy without a prescription.
- you are breastfeeding, pregnant or trying to become pregnant.
If you take Daraprim tablets while you are pregnant, folic acid supplementation may be required.
How do I take Daraprim tablets?
How much to take
Take Daraprim tablets as directed by your doctor or pharmacist.
Your doctor will tell you how many tablets to take and how often to take them. You will also find this information on the label of your medicine.
How to take it
Take Daraprim tablets with a glass of water.
How long to take it for
Your doctor will tell you how long to take Daraprim tablets.
Do not stop taking Daraprim tablets, or change the dose without first checking with your doctor.
What do I do if I take too much? (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, if you think you or anyone else may have taken too much Daraprim tablets, even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking Daraprim tablets
Things you must do
Tell your doctor if, for any reason, you have not taken your medicine exactly as directed.
Otherwise, your doctor may think that it was not working as it should and change your treatment unnecessarily.
Things you must not do
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Do not use Daraprim tablets to treat any other complaints unless your doctor says to.
Things to be careful of
Be careful driving or operating machinery until you know how Daraprim tablets affects you.
What are the side effects?
Check with your doctor as soon as possible if you think you are experiencing any side effects or allergic reactions due to taking Daraprim tablets, even if the problem is not listed below.
Like other medicines, Daraprim tablets can cause some side effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and need medical attention.
The most commonly reported side effects:
- nausea
- colic
- vomiting
- diarrhoea
- blood abnormalities.
Less commonly reported side effects:
- headache
- giddiness
- dry mouth or throat
- fever
- a feeling of general discomfort
- dermatitis
- abnormal colour of the skin
- depression.
Tell your doctor immediately if you notice any of the following:
- Wheezing, swelling of the lips/mouth, difficulty in breathing, hay fever, lumpy rash (hives) or fainting. These could be a symptom of an allergic reaction.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
How do I store Daraprim tablets?
Keep this medicine where children cannot reach it, such as in a locked cupboard.
Keep Daraprim tablets in a cool place, where they stay below 30°C and are protected from light.
Do not leave in a car, on window sill or in bathroom.
Keep Daraprim tablets in the blister pack until it is time to take them.
Return any unused or expired medicine to your pharmacist.
Product description
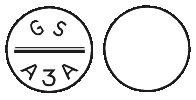
What Daraprim tablets look like
Daraprim tablets are white, scored and marked "GS A3A".
Daraprim tablets are available in blister packs containing 50 tablets.
Ingredients
Daraprim tablets contain the active ingredient pyrimethamine 25mg.
Daraprim tablets also contain lactose, maize starch, hydrolysed starch, docusate sodium and magnesium stearate.
Daraprim tablets contain lactose.
Supplier
Your Daraprim tablets are supplied by:
Arrow Pharma Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Where to go for further information
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you advice on the treatment of your condition.
This leaflet was prepared on April 2016.
Daraprim tablets: AUST R 97456.


 Treatment should be given for 3 to 6 weeks. If further therapy is indicated, a period of 2 weeks should elapse between treatments.
Treatment should be given for 3 to 6 weeks. If further therapy is indicated, a period of 2 weeks should elapse between treatments.