SUMMARY CMI
DURIDE®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using DURIDE?
DURIDE contains the active ingredient isosorbide mononitrate. DURIDE is used to prevent angina.
For more information, see Section 1. Why am I using DURIDE? in the full CMI.
2. What should I know before I use DURIDE?
Do not use if you have ever had an allergic reaction to DURIDE or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use DURIDE? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with DURIDE and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use DURIDE?
- Your doctor will decide the right dose for you.
- The usual dose is one tablet once a day.
More instructions can be found in Section 4. How do I use DURIDE? in the full CMI.
5. What should I know while using DURIDE?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using DURIDE? in the full CMI.
6. Are there any side effects?
Tell your doctor or pharmacist if you notice any of the following and they worry you: headache, dizziness, light-headedness or feeling faint, feeling sick (nausea), rapid heart beat, poor appetite, vomiting, heartburn, diarrhoea, rash, itching, muscle tenderness or weakness not caused by exercise, tiredness or sleep disturbances.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
DURIDE®
Active ingredient(s): isosorbide mononitrate
Consumer Medicine Information (CMI)
This leaflet provides important information about using DURIDE. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using DURIDE.
Where to find information in this leaflet:
1. Why am I using DURIDE?
2. What should I know before I use DURIDE?
3. What if I am taking other medicines?
4. How do I use DURIDE?
5. What should I know while using DURIDE?
6. Are there any side effects?
7. Product details
1. Why am I using DURIDE?
DURIDE contains the active ingredient isosorbide mononitrate. DURIDE belongs to a group of medicines called nitrates. These medicines work by widening blood vessels, letting more blood and oxygen reach the heart.
DURIDE is used to prevent angina.
Angina is a pain or uncomfortable feeling in the chest, often spreading to the arms or neck and sometimes to the shoulders and back. This may be caused by too little blood and oxygen getting to your heart. The pain of angina is usually brought on by exercise or stress.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
This medicine is not addictive.
There is not enough information to recommend the use of this medicine for children.
2. What should I know before I use DURIDE?
Warnings
Do not use DURIDE if:
- you are allergic to any medicine containing isosorbide mononitrate or any of the ingredients listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Always check the ingredients to make sure you can use this medicine. - you are taking medicines used to treat impotence, including sildenafil (Viagra), tadalafil (Cialis) and vardenafil (Levitra).
- you have:
- low blood pressure
- experienced shock, including it being caused by low blood pressure or heart failure
- swelling of the lining that surrounds the heart (pericarditis)
- a weakened heart muscle - the expiry date printed on the pack has passed or the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
Do not take DURIDE to relieve acute angina.
This medicine should only be used for the prevention of angina.
Do not give this medicine to children.
Safety and effectiveness in children have not been established.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Check with your doctor if you:
- have allergies to any other medicines, foods, preservatives or dyes
- have or have had any of the following medical conditions:
- liver problems
- kidney problems
- low blood pressure (this can make you feel faint, weak or dizzy, especially when you stand up suddenly)
- heart or blood vessel disease - are or have been an industrial worker, as you may have had long-term exposure to organic nitrates, which may affect how your body responds to DURIDE.
If you have not told your doctor about any of the above, tell them before you start taking DURIDE.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Do not take this medicine if you are pregnant.
It may affect your developing baby if taken during pregnancy.
Do not breast-feed if you are taking this medicine unless your doctor says it is safe.
It is not known whether DURIDE is passed into breast milk, therefore it is not recommended if you are breastfeeding.
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding.
Your doctor can discuss with you the risks and benefits involved.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and DURIDE may be interfere with each other. These include:
- medicines used to treat high blood pressure and heart conditions, such as calcium antagonists
- medicines used to treat liver disease, such as propranolol
- medicines used to treat impotence in men, such as sildenafil (Viagra), tadalafil (Cialis) and vardenafil (Levitra)
These medicines may be affected by DURIDE or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect DURIDE.
4. How do I use DURIDE?
Follow all directions given to you by your doctor and pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
- The usual dose is one tablet once a day.
- Some patients may need a different dose.
- Your doctor will decide the right dose for you.
How to take it
- Swallow the tablets whole with half a glass of water.
DURIDE tablets can be broken in half if care is taken not to crumble them. Break the tablet in half with care if your doctor has prescribed half a tablet. - Do not crush or chew the tablets.
DURIDE tablets are modified release tablets. This means that the medicine is released into the blood over an extended period of time. If you crush or chew the tablets, they will not work as well.
When to take it
- Take your medicine at about the same time each day.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it. - It does not matter if you take this medicine before or after food.
- If your doctor tells you to take two tablets each day, take both tablets at the same time.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your angina but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take DURIDE
If it is more than 8 hours since you should have taken your dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you miss a dose of DURIDE, you may get attacks of angina.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you use too much DURIDE
If you think that you have used too much DURIDE, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
If you take too much DURIDE, you may experience a headache, a fast heart beat and feel dizzy or faint. You may feel excited, flushed, have cold sweats, feel sick or vomit.
5. What should I know while using DURIDE?
Things you should do
- If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking DURIDE.
- Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
- If you become pregnant while taking this medicine, tell your doctor immediately.
- Keep all of your doctor's appointments so that your progress can be checked.
- If you continue to have angina attacks, or if they become frequent while you are taking DURIDE tell your doctor.
Things you should not do
- Do not use DURIDE to relieve acute attacks of angina.
This medicine is used to prevent angina. Your doctor will have given you other medicines to use if you have an angina attack. - Do not take DURIDE to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not stop taking your medicine, or change the dose, without checking with your doctor.
If you stop taking it suddenly, your angina may worsen, or you may have unwanted side effects.
Things to be careful of
You will probably feel better when you start to take DURIDE but be careful not to overdo physical activities straight away.
You will need time to improve your physical fitness.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how DURIDE affects you.
This medicine may cause dizziness, light-headedness or fainting in some people. If any have any of these symptoms, do not drive, operate machinery or do anything that could be dangerous.
Drinking alcohol
Be careful when drinking alcohol while taking DURIDE.
Combining DURIDE and alcohol can make you more sleepy, dizzy or lightheaded, due to a drop in your blood pressure.
After using DURIDE
Be aware that the outer shell of the DURIDE tablet passes through the body. This does not affect the way it works.
DURIDE tablets are made of a wax-like shell which does not dissolve in the body. After the active ingredient, isosorbide mononitrate, has been absorbed into the body, you may see this empty tablet shell in your faeces (bowel motions).
Looking after your medicine
Keep your tablets in the pack until it is time to take them.
If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store DURIDE or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If your doctor tells you to stop taking this medicine, or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
6. Are there any side effects?
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking DURIDE.
This medicine helps most people with angina, but it may have unwanted side effects in some people. All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Side effects
| Side effects | What to do |
| Tell your doctor or pharmacist if you notice any of the following and they worry you: |
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What DURIDE contains
| Active ingredient (main ingredient) | isosorbide mononitrate 60 mg |
| Other ingredients (inactive ingredients) | aluminium silicate colloidal anhydrous silica magnesium stearate microcrystalline cellulose Opadry Yellow OY-LS-22814 (ARTG PI No: 2828) Paraflint Med 851 (ARTG PI No: 2858) |
| Potential allergens | DURIDE contains lactose and sulfites. |
Do not take this medicine if you are allergic to any of these ingredients.
What DURIDE looks like
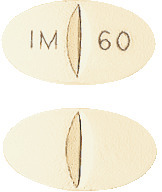
DURIDE is a yellow, film coated, elliptical shaped tablet, 13.1 x 7.1 mm, scored on both sides and imprinted "IM I 60" on one side. Each pack contains 30 tablets. (AUST R 60907).
Who distributes DURIDE
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in July 2022.
DURIDE_cmi\Jul22/00
Published by MIMS September 2022

 Chemical name: 1,4:3,6-dianhydro-D-glucitol 5-nitrate.
Chemical name: 1,4:3,6-dianhydro-D-glucitol 5-nitrate.