SUMMARY CMI
ELEVA®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about taking this medicine, speak to your doctor or pharmacist.
1. Why am I taking ELEVA?
ELEVA contains the active ingredient sertraline hydrochloride. ELEVA is used to treat depression and conditions called obsessive compulsive disorder (OCD), panic disorder, social phobia (social anxiety disorder) and premenstrual dysphoric disorder (PMDD). For more information, see Section 1. Why am I taking ELEVA? in the full CMI.
2. What should I know before I take ELEVA?
Do not take if you have ever had an allergic reaction to sertraline or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I take ELEVA? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with ELEVA and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take ELEVA?
Your doctor will tell you how many tablets you need to take each day. This may depend on your age, your condition and whether or not you are taking any other medicines. More instructions can be found in Section 4. How do I take ELEVA? in the full CMI.
5. What should I know while taking ELEVA?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking ELEVA? in the full CMI.
6. Are there any side effects?
Speak to your doctor or pharmacist if you have: headache, dizziness, shaking or tremors, unusually overactive, muscle stiffness or weakness, decrease or loss of touch or other senses, sleepiness, drowsiness, impaired concentration, vision disturbance.
Call your doctor straight away if you have: agitation, nervousness, anxiety, frightening dreams, yawning, abnormal thinking, teeth grinding, uncontrollable muscle spasms affecting the eyes, head, neck and body, temporary paralysis or weakness of muscles, lockjaw, painful, swollen joints, difficulty in breathing, wheezing or coughing, uncontrollable movements of the body.
Tell your doctor immediately or go straight to Emergency at your nearest hospital if you have: fits or seizures, signs of allergy such as rash or hives, swelling of the face, lips or tongue, wheezing or difficulty breathing, symptoms of sudden fever with sweating, fast heartbeat and muscle stiffness, which may lead to loss of consciousness, thoughts of suicide or self-harm.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
ELEVA®
Active ingredient: sertraline hydrochloride
Consumer Medicine Information (CMI)
This leaflet provides important information about taking ELEVA. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about taking ELEVA.
Where to find information in this leaflet:
1. Why am I taking ELEVA?
2. What should I know before I take ELEVA?
3. What if I am taking other medicines?
4. How do I take ELEVA?
5. What should I know while taking ELEVA?
6. Are there any side effects?
7. Product details
1. Why am I taking ELEVA?
ELEVA contains the active ingredient sertraline hydrochloride. ELEVA belongs to a group of medicines called selective serotonin reuptake inhibitors (SSRIs). They are thought to work by blocking the uptake of a chemical called serotonin into nerve cells in the brain. Serotonin and other chemicals called amines are involved in controlling mood.
ELEVA is used to treat depression and conditions called obsessive compulsive disorder (OCD), panic disorder, social phobia (social anxiety disorder) in adults and premenstrual dysphoric disorder (PMDD) in women.
PMDD affects some women in the days before their period. PMDD is different from premenstrual syndrome (PMS). The mood symptoms (anger, sadness, tension, etc) in PMDD are more severe than in PMS and affect the woman's daily activities and relationships with others.
ELEVA should not be used in children and adolescents under the age of 18 years for the treatment of any medical condition other than obsessive compulsive disorder (OCD). The safety and efficacy of ELEVA for the treatment of medical conditions (other than OCD) in this age group have not been satisfactorily established.
For the treatment of OCD, ELEVA is not recommended for use in children under the age of 6, as the safety and efficacy in children of this age group has not been established.
There is no evidence that ELEVA is addictive.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
2. What should I know before I take ELEVA?
Warnings
Do not take ELEVA if:
- you are allergic to sertraline, or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Always check the ingredients to make sure you can take this medicine. - you have epilepsy not properly controlled by medication.
- you are taking another medicine for depression called a monoamine oxidase inhibitor (MAOI) or have been taking it within the last 14 days. Taking ELEVA with a MAOI (e.g. Aurorix, Eldepryl, Nardil, Parnate) may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and convulsions (fits).
- you are taking:
- phentermine, a medicine used to help weight loss
- tryptophan, an amino acid which may be found in protein-based foods, some sports and dietary supplements or multivitamin preparations
- methadone, a medicine used to treat drug addiction
- medicines used to treat migraine, e.g. sumatriptan (Imigran)
- dextromethorphan, a medicine used as a cough suppressant in some cold and flu medications
- pimozide, a medicine used to treat disturbances in thinking, feeling and behaviour
- medicines used for pain management such as fentanyl, tapentadol (Palexia), tramadol or pethidine.
These medicines can cause an exaggerated response to ELEVA.Ask your doctor or pharmacist if you are not sure if you have been taking one of these medicines.
Do not give ELEVA to:
- children or adolescents under the age of 18 unless the doctor has prescribed it for the treatment of OCD.
- children under the age of 6 for the treatment of OCD.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should be taking ELEVA, talk to your doctor.
Check with your doctor if you:
- have or have had any of the following medical conditions:
- any other mental illness
- epilepsy or seizures
- liver or kidney problems
- heart conditions causing irregular heartbeats
- a tendency to bleed more than normal
- diabetes mellitus
- glaucoma, an eye condition - are pregnant or intend to become pregnant
- are breastfeeding or wish to breastfeed
- take any medicines for any other conditions.
Tell your doctor if you have allergies to any foods, dyes, preservatives or any other medicines.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
There have been reports that babies exposed to ELEVA and other antidepressants during the third trimester of pregnancy may develop complications immediately after birth.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
ELEVA passes into breast milk and may affect your baby.
Your doctor will discuss the risks and benefits of taking ELEVA when pregnant or breastfeeding.
If you have not told your doctor about any of the above, tell them before you start taking ELEVA.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including all medicines, vitamins, natural therapies or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with ELEVA and affect how it works.
Tell your doctor or pharmacist if you are taking any of the following medicines:
- MAOIs (monoamine oxidase inhibitors), a group of medicines used to treat depression and the symptoms of Parkinson's disease
Taking ELEVA with a MAOI, or within 14 days of stopping a MAOI may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and convulsions (fits). - other MAOI drugs such as linezolid, an antibiotic used to treat pneumonia and certain skin infections
- other medicines for depression, panic disorder, social anxiety disorder or obsessive illnesses (e.g. dosulepin (Dothep), fluoxetine (Prozac), paroxetine (Aropax), citalopram (Cipramil), venlafaxine (Efexor XR))
- lithium (e.g. Lithicarb), a medicine used to treat mood swings
- other medicines for PMDD (e.g. Prozac)
- tryptophan (contained in protein-based foods or dietary proteins)
- phentermine (weight-reducing medicines)
- dextromethorphan (used in cold and flu medicines to suppress cough)
- medicines for strong pain management such as fentanyl, tapentadol (Palexia), tramadol or pethidine
- methadone, a medicine used to treat drug addiction
- other medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis (e.g. aspirin or NSAIDs such as ibuprofen or diclofenac)
- metamizole, which is an inducer of metabolising enzymes, as this may cause a reduction in plasma concentrations of sertraline thus efficacy
- pimozide, a medicine used to treat disturbances in thinking, feeling and behaviour
- St John's wort, a herbal remedy used to treat mood disorders
- medicines for treating psychotic illness such as clozapine (e.g. Clozaril) which is used to treat schizophrenia
- flecainide (e.g. Tambocor), a medicine used to treat irregular heartbeats
- warfarin (e.g. Marevan, Coumadin) or other medicines used to prevent blood clots
- phenytoin (e.g. Dilantin), a medicine used to treat epilepsy
- sumatriptan (e.g. Imigran), a medicine used to treat migraine
- diazepam or other medicines that act on the brain or nervous system (e.g. Serepax, Valium)
- cimetidine, a medicine used to treat reflux and ulcers
- tolbutamide, a medicine used to treat diabetes
- dexamphetamine and lisdexamfetamine, medicines used to treat Attention Deficit Hyperactivity Disorder (ADHD)
- antibiotics.
These medicines may be affected by ELEVA or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
You should wait at least 14 days after stopping ELEVA before starting any other medicines for depression or obsessive illnesses from the MAOI group, such as Aurorix (moclobemide), Eldepryl (selegiline), Nardil (phenelzine), and Parnate (tranylcypromine).
All of the above precautions are important even after you have stopped taking ELEVA.
The effects of ELEVA may last for some days after you have stopped taking it.
Not all brand names are given for the medicines listed above. Your doctor or pharmacist has more information on these medicines or other medicines to be careful with or avoid while taking ELEVA.
If you have not told your doctor or pharmacist about these things, tell them before you start taking ELEVA.
Some combinations of medicines may increase the risk of serious side effects and are potentially life-threatening.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect ELEVA.
4. How do I take ELEVA?
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how many tablets you need to take each day. This may depend on your age, your condition and whether or not you are taking any other medicines.
- Depression - Adults
The usual starting dose is one 50 mg tablet each day. Your doctor may increase the dose gradually up to 200 mg a day if necessary. - Obsessive Compulsive Disorder - Children (6-12 years)
The usual starting dose is 25 mg each day which is half a 50 mg tablet. Your doctor may increase the dose to 50 mg per day after one week. - Obsessive Compulsive Disorder - Adults and Adolescents (13-18 years) and Adults
The usual starting dose is one 50 mg tablet each day. - Panic Disorder - Adults
The usual starting dose is 25 mg day each day which is half a 50 mg tablet. Your doctor may increase the dose to 50 mg per day after one week. - Social Phobia (Social Anxiety Disorder) - Adults
The usual starting dose is 25 mg each day which is half a 50 mg tablet. Your doctor may increase the dose to 50 mg per day after one week.
Do not take more than 200 mg a day for the conditions listed above.
- Premenstrual Dysphoric Disorder (PMDD)
- If taking throughout the menstrual cycle
The usual starting dose is one 50 mg tablet a day. This may be increased to a maximum of 150 mg a day if needed. Increase the dose in a step wise fashion. If you are unclear how to do this ask your pharmacist or doctor for advice.
- If taking ELEVA in the last 14 days of the menstrual cycle
-- The usual starting dose is one 50 mg tablet a day. This may be increased to a maximum of 100 mg a day.
-- Do not take more than the maximum doses recommended above for PMDD. - Follow the instructions provided and take ELEVA until your doctor tells you to stop.
When to take ELEVA
- Try to take your tablet at about the same time each day.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it. - ELEVA can be taken with or without food.
- For women with PMDD, your doctor may ask you to take this medicine only at certain times of the month.
How to take ELEVA
- Swallow the tablets with a glass of water.
How long to take ELEVA
- Most medicines for depression and obsessive illnesses take time to work so do not be discouraged if you do not feel better straight away.
- It may take 2 to 4 weeks or even longer to feel the full benefit of ELEVA.
- Continue taking your medicine for as long as your doctor tells you to.
- Even when you feel well, you may need to take ELEVA for several months or even longer.
- If you have PMDD, your doctor may ask you to take this medicine only at certain times of the month.
- Do not stop taking ELEVA, or change the dose, without first checking with your doctor.
- Occasionally the symptoms of depression or other psychiatric conditions may include thoughts of harming yourself or committing suicide. It is possible that these symptoms may continue or increase until the full anti-depressant effect of your medicine becomes apparent (i.e. one to two months).
- You or anyone close to you or caring for you should watch for these symptoms and tell your doctor immediately or go to the nearest hospital if you have any distressing thoughts or experiences during this initial period or at any other time.
- Contact your doctor if you experience any worsening of your depression or other symptoms at any time during your treatment.
If you forget to take ELEVA
ELEVA should be taken regularly at the same time each day. If you miss your dose at the usual time and if it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much ELEVA
If you think that you or anyone else have taken too much ELEVA, urgent medical attention may be needed.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of an overdose may include:
- feeling drowsy
- nausea, diarrhoea, vomiting
- fast or irregular heartbeats
- tremors
- feeling agitated or dizzy.
5. What should I know while taking ELEVA?
Things you should do
- Tell your doctor or pharmacist that you are taking ELEVA if you are about to be started on any new medicines.
- Tell the surgeon or anaesthetist that you are taking this medicine if you are going to have surgery.
It may interact with other medicines used during surgery and cause unwanted side effects. - Keep all of your doctor's appointments so that your progress can be checked.
- Tell your doctor that you are taking this medicine if you are about to have any urine tests. It may interfere with the results of some tests.
Call your doctor straight away if you:
- become pregnant while taking ELEVA. Do not stop taking this medicine until you have spoken to your doctor.
If you are a woman of child-bearing age, you should avoid become pregnant while taking ELEVA. - have any suicidal thoughts or other mental/mood changes.
A worsening of depressive symptoms including thoughts of suicide or self-harm may occur in the first one or two months of you taking ELEVA or when the doctor changes your dose. These symptoms should subside when the full effect of ELEVA takes place.
Children, adolescents or young adults under 24 years of age are more likely to experience these effects during the first few months of treatment.
Patients and caregivers should be alert and monitor for these effects.
Signs and symptoms of suicide include:
- thoughts or talk of death or suicide
- thoughts or talk of self-harm or harm to others
- any recent attempts of suicide or self-harm
- increase in aggressive behaviour, irritability or agitation
- worsening of depression.
All mention of suicide or violence must be taken seriously.
If you or someone you know is demonstrating these warning signs of suicide while taking ELEVA, contact your doctor or a mental health professional right away.
Children should have regular check-ups with the doctor to monitor growth and development.
Remind any doctor, dentist or pharmacist you visit that you are taking ELEVA.
Things you should not do
- Do not suddenly stop taking your medicine, or change the dose, without checking with your doctor.
- Suddenly stopping ELEVA may cause dizziness, light headedness, numbness, unusual tingling feelings or shakiness.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not take ELEVA to treat any other complaints unless your doctor tells you to.
- Do not let yourself run out of tablets over the weekend or on holidays.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how ELEVA affects you.
Some medicines for depression may affect your ability to drive or operate machinery or do anything else that could be dangerous if you are not alert.
Children should be careful when riding bicycles or climbing trees.
Drinking alcohol
Tell your doctor if you drink alcohol.
Although drinking moderate amounts of alcohol is unlikely to affect your response to ELEVA, your doctor may suggest avoiding alcohol while you are taking ELEVA.
If you are feeling drowsy or are uncoordinated, be careful that you do not fall over.
ELEVA, like other medicines in this class, may increase your risk of bone fracture.
Looking after your medicine
- Keep your tablets in a cool dry place where the temperature stays below 25°C.
- Keep your tablets in the pack until it is time to take them.
If you take the tablets out of the pack they may not keep well.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to take this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not take this medicine after the expiry date.
6. Are there any side effects?
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ELEVA.
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
It can be difficult to tell whether side effects are the result of taking ELEVA, effects of your condition or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the list of side effects. You may not experience any of them.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor or pharmacist if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away if you notice any of these serious side effects. |
Very Serious side effects
| Very Serious side effects | What to do |
The following symptoms are signs of side effects named Serotonin Syndrome (SS) or Neuroleptic Malignant Syndrome (NMS). SS is caused by medications which build up high levels of serotonin in the body. NMS is a life-threatening emergency associated with the use of antipsychotic medicines. The risk of SS and NMS with SSRI's is increased with combined use of other SSRIs, MAOIs and other antipsychotic medicines.
| Tell you doctor immediately or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. You may need urgent medical attention or hospitalisation. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Some of these side effects (e.g., changes in thyroid function, liver function or glucose control) can only be found when your doctor does tests from time to time to check your progress.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What ELEVA contains
| Active ingredient (main ingredient) | sertraline 50 mg or 100 mg per tablet |
| Other ingredients (inactive ingredients) |
|
Do not take this medicine if you are allergic to any of these ingredients.
What ELEVA looks like
ELEVA 50: 4.2-4.5 mm x 10.3-10.7 mm, white to off-white, capsule shaped, film-coated tablets with “ST|50” on one side and "G" on the other side. (AUST R 95581 and AUST R 95582)
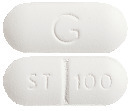
ELEVA 100: 5.2-5.6 mm x 13.0-13.4 mm, white to off-white, capsule shaped, film-coated tablets with “ST|100” on one side and "G" on the other side. (AUST R 95583 and AUST R 95584)
Each blister pack contains 30 tablets.
Who distributes ELEVA
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in December 2023.
ELEVA® is a Viatris company trade mark
ELEVA_cmi\Dec23/00
Published by MIMS February 2024



 Chemical name: (1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride.
Chemical name: (1S,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine hydrochloride.