What is in this leaflet
This leaflet answers some common questions about ENLAFAX-XR capsule.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking ENLAFAX-XR against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ENLAFAX-XR is used for
ENLAFAX-XR capsules contain an active ingredient called "venlafaxine hydrochloride".
This medicine is used in the treatment and prevention of relapse of depression and in the treatment of social phobia (social anxiety disorder).
It belongs to a class of medications for depression and anxiety, called Serotonin-Noradrenaline Reuptake Inhibitors (SNRIs).
Serotonin and noradrenaline are chemical messengers that allow certain nerves in brain to work. ENLAFAX-XR capsules increase the level of these two messengers. Experts think this is how it helps to restore your feeling of wellness.
Depression is longer lasting or more severe than the 'low moods' that everyone has from time to time. It is thought to be caused by a chemical imbalance in parts of the brain. This imbalance affects your whole body and can cause emotional and physical symptoms such as feeling low in spirit, lose interest in usual activities, be unable to enjoy life, have poor appetite or over eat, have disturbed sleep, often waking up early, loss of sex drive, low energy and feel guilty over nothing.
Social phobia is a fear of being embarrassed, judged or evaluated negatively in social situations, which can result in substantial impairment in the person's social emotional, interpersonal and occupational life.
Depression and anxiety are treatable illnesses. Anxiety and tension associated with the normal stress of daily life usually does not require treatment with medicines.
There is no evidence that ENLAFAX-XR is addictive.
Ask your doctor if you have any questions about why ENLAFAX-XR has been prescribed for you. Your doctor may have prescribed ENLAFAX-XR capsules for another purpose.
Before you take ENLAFAX-XR
When you must not take it
Do not take ENLAFAX-XR if you are taking other medications for depression known as monoamine oxidase inhibitors, even if you have stopped taking them now, but have taken them within the last 14 days.
Do not take ENLAFAX-XR if you are allergic to it or to any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- Skin rash
- Itching or hives on the skin
- Swelling of the face, lips, tongue or other parts of the body
- Shortness of breath, wheezing or troubled breathing; difficulty swallowing
Do not give ENLAFAX-XR to children or adolescents under 18 years of age. The safety and effectiveness of ENLAFAX-XR in this age group have not been established.
Do not take ENLAFAX-XR after the expiry date printed on the pack. If you take this medicine after the expiry date has passed, it may not work as well as it should.
Do not take ENLAFAX-XR if the packaging is torn or show signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor or pharmacist if you are allergic to the active ingredient or any of the other ingredients listed at the end of this leaflet.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant. ENLAFAX-XR is not recommended for use during pregnancy. Your doctor will discuss the risks and benefits of using ENLAFAX-XR if you are pregnant. One of these risks is that newborn babies whose mothers have been taking ENLAFAX-XR in the last few months of pregnancy, may experience problems soon after delivery, including breathing difficulties, seizures and lack of oxygen in their blood.
If you take ENLAFAX-XR or similar anti-depressants mid to late in your pregnancy, you may develop a condition known as "pre-eclampsia", which is characterised by persistent high blood pressure during or after pregnancy. Symptoms of pre-eclampsia can include headaches, abdominal pain, shortness of breath or burning around the sternum, nausea and vomiting, confusion, heightened state of anxiety, and/or visual disturbances such as oversensitivity to light, blurred vision, or seeing flashing spots or auras.
If you take ENLAFAX-XR or similar antidepressants in the last month of your pregnancy, you may experience heavy bleeding during and/or after delivery.
Continuing treatment with ENLAFAX-XR or similar antidepressants during pregnancy should be strictly as directed by your doctor. Symptoms of a relapse may occur if treatment is discontinued, even if major depression was previously under control.
Tell your doctor or pharmacist if you are breast-feeding or planning to breast-feed. ENLAFAX-XR passes into breast milk and there is a possibility that the breast-fed baby may be affected. For this reason, the use of ENLAFAX-XR is not recommended in breast-feeding women.
Tell your doctor if you have or have had any, medical conditions, especially the following:
- A history of fits (seizures or convulsions)
- A personal history or family history of bipolar disorder
- A history of aggression
- Blood pressure problems
- Glaucoma (increased pressure in the eye)
- A tendency to bleed more than normal or you are taking medicines to prevent blood clots
- A history of restlessness or difficulty sitting still
- Diabetes
- Raised cholesterol levels or you are taking medicines to lower cholesterol
- Problems with your kidneys or liver
- Problems with your heart, especially conditions causing irregular heartbeats.
Your doctor may wish to do some heart tests such as an electrocardiogram (ECG) or blood tests during treatment with ENLAFAX-XR.
Tell your doctor if you plan to have surgery.
If you have not told your doctor about any of the above, tell them before you take ENLAFAX-XR.
Taking other medicines
Tell your doctor or pharmacist if you take any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop, such as St John's Wort or tryptophan supplements.
Do not start to take any other medicine while you are taking ENLAFAX-XR, unless it is prescribed or approved by your doctor.
Some medicines and ENLAFAX-XR may interfere with each other. These include:
- Medications for depression known as monoamine oxidase inhibitors (such as moclobemide, linezolid, phenelzine and tranylcypromine), even if you have stopped taking them now, but have taken them within the last 14 days. Your doctor or pharmacist can tell you what to do if you are taking any of these medicines.
- Any other medications for depression, anxiety, obsessive-compulsive disorder or premenstrual dysphoric disorder, including St. John's Wort.
- Haloperidol, risperidone, lithium or clozapine (used to treat mental disorders).
- Triptans (used to treat migraine).
- Cimetidine (used to treat reflux and stomach ulcers)
- Amiodarone or quinidine (used to treat irregular heartbeats)
- Tramadol, fentanyl, tapentadol, pethidine, methadone and pentazocine (a pain killer).
- Amphetamines (used to treat attention deficit hyperactivity disorder or ADHD)
Your doctor may wish to do some heart tests such as an electrocardiogram (ECG) or blood tests if you are using either of these medicines whilst taking ENLAFAX-XR.
- Medicines used to prevent blood clotting such as anti-coagulants and platelet inhibitors (e.g. warfarin).
- Indinavir (an antiviral)
- Medications for weight loss, including sibutramine
- Erythromycin and linezolid (used to treat infections)
- Ketoconazole or fluconazole (used as antifungal medicines)
- Metoprolol (used to treat high blood pressure or angina).
These medicines may be affected by ENLAFAX-XR or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
You doctor or pharmacist has more information on medicines to be careful with or to avoid while taking ENLAFAX-XR.
How to take ENLAFAX-XR
DO NOT DIVIDE, CRUSH, CHEW OR DISSOLVE.
ENLAFAX-XR capsules are to be swallowed whole and are not be divided, chewed or crushed. Taking divided, chewed or crushed ENLAFAX-XR capsules, in theory, could lead to the rapid release and absorption of venlafaxine.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
The usual starting dose is 75mg taken once daily. If necessary, after two weeks, your doctor may increase your dose.
Do not change dose unless your doctor tells you to.
If you have kidney or liver problems, you may need a lower dose of ENLAFAX-XR.
If you have heart problems and your doctor wishes to increase your dose of ENLAFAX-XR, your doctor may first do some blood tests or heart tests such as an electrocardiogram (ECG).
When to take it
ENLAFAX-XR should be taken once daily with food, at approximately the same time each day. This could be either in the morning of in the evening.
Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Avoid drinking alcohol while you are using ENLAFAX-XR.
Your doctor may have switched your treatment from Venlafaxine Hydrochloride tablets to ENLAFAX-XR capsules. If this happens, your doctor will normally prescribe a dose that is equivalent to your total daily dose of tablets. The dose of ENLAFAX-XR capsules should be taken only once daily.
If you forget to take it
If it is less than 12 hours until your next dose, skip the dose you missed and then take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking as you would normally.
Do not take a double dose to make up for the dose you missed. Contact your doctor if you have missed more than two doses in a row.
Always finish the capsules you are taking in the current pack before you start a new pack.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
How long to take it for
The length of treatment will depend on how quickly your symptoms improve. Most antidepressants take time to work, so don't be discouraged if you don't feel better right away. Some of your symptoms may improve in 1 or 2 weeks but it can take up to another few weeks to feel any real improvement.
Even when you feel well, you will usually have to take ENLAFAX-XR for several months or even longer to make sure the benefits will last. Discuss this with your doctor and don't stop taking ENLAFAX-XR until gaining your doctor's agreement.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much ENLAFAX-XR. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention. Keep telephone numbers for these places handy. Take the pack of capsules/tablets with you to the doctor or hospital.
If you take too many ENLAFAX-XR capsules you may:
- Feel sleepy
- Vomit
- Have an increased heart rate or changes in heart rhythm
- Have a seizure (fits)
- Have breathing difficulties
- Become unconscious
- Have dilated pupils.
While you are taking ENLAFAX-XR
Things you must do
Visit your doctor regularly for a check up so that your progress can be checked. Your doctor may do some tests (such as an electrocardiogram (ECG) or blood tests) from time to time to make sure the medicine is working and to prevent unwanted side effects.
Always discuss any questions you have about ENLAFAX-XR capsules with your doctor.
If you are going to have a surgery, tell your doctor that you are taking this medicine. Some agents used to assist your doctor during surgery may interact with ENLAFAX-XR leading to unwanted side effects.
If you are about to have any urine tests, tell your doctor that you are taking this medicine. ENLAFAX-XR may interfere with the results of some tests.
Take ENLAFAX-XR capsules as your doctor has prescribed.
Keep enough ENLAFAX-XR capsules to last weekends and holidays. You need a prescription from your doctor to get more ENLAFAX-XR capsules.
Watch carefully for signs that your depression or anxiety is getting worse, especially in the first few weeks of treatment, or if your dose has changed. Sometimes people with depression can experience a worsening of their depressive symptoms. This can happen even when taking an antidepressant.
Information from clinical trials has suggested that children, adolescents and young adults (18-24 years), particularly those with depression, may be at increased risk of suicidal behaviour (including suicide attempts) when treated with ENLAFAX-XR, especially during initial treatment.
Tell your doctor immediately if you experience any of the following symptoms, especially if they are severe, you have not had these symptoms before or they happen very suddenly.
- Anxiety or agitation
- Panic attacks
- Difficulty sleeping
- Irritability
- Aggressiveness
- Hostility or impulsiveness
- Restlessness
- Overactivity or uninhibited behaviour
- Other unusual changes in behaviour
- Thoughts of suicide.
Tell your doctor immediately if you have any thoughts about suicide or doing harm to yourself.
Warning signs of suicide
All thoughts or talk about suicide or violence are to be taken seriously. If you or someone you know is showing the following warning signs, either contact your doctor or a mental health advisor right away or go to the nearest hospital for treatment:
- Thoughts or talk about death or suicide
- Thoughts or talk about self-harm or doing harm to others
- Any recent attempts of self-harm
- An increase in aggressive behaviour, irritability or agitation.
Things you must not do
Do not suddenly stop taking ENLAFAX-XR or lower the dose suddenly, without first checking with your doctor. Check with your doctor for the best way to slowly reduce the amount of ENLAFAX-XR you are taking before stopping completely.
Side effects from stopping treatment with ENLAFAX-XR may include:
- Headache
- Nausea and vomiting
- Dizziness
- Insomnia
- Nervousness
- Anxiety
- Confusion and agitation
- Diarrhoea
- Sweating
- Loss of appetite
- Tremor
- Flu-like symptoms
- Impaired coordination and balance
- Tingling or numbness of the hands and feet.
Slowly reducing the amount of ENLAFAX-XR being taken reduces the possibility of these effects occurring.
Some of these symptoms may impair driving, or the operation of dangerous machinery. These activities should be avoided if you experience these symptoms.
Do not take ENLAFAX-XR to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating dangerous machinery until you know how it affects you. ENLAFAX-XR capsules may make you feel drowsy.
If you are feeling drowsy or are uncoordinated, be careful that you do not fall over. ENLAFAX-XR, like other medicines in this class, may increase your risk of bone fracture.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ENLAFAX-XR.
This medicine helps most people with depression, but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking this medicine, effects of your condition, or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following side effects and they worry you:
- Stomach, bowel or urinary tract problems such as:
- Nausea or vomiting
- Loss of appetite
- Diarrhoea
- Constipation
- Difficulty passing urine. Passing urine more frequently or urinary incontinence. - Changes in your behaviour such as:
- Difficulty sleeping or abnormal dreams
- Paranoia
- Sexual function problems such as delayed ejaculation, problems with erection, decreased sex drive or difficulties achieving orgasm.
- Nervousness
- Teeth grinding
- Impaired coordination and balance - Difficulty thinking or working because of:
- Yawning
- Feeling sedated or drowsy
- Fainting or dizziness after standing up
- Restlessness or difficulty sitting still
- Headache
- Rapid heart beat
- Heavy or irregular menstrual periods. - Changes in your appearance such as:
- Sweating (including night sweats)
- Hot flushes
- Rash
- Hair loss
- Itchiness
- Weight loss or weight gain
- Flow of milk in women who are not breastfeeding
- Blurred vision
- Ringing in the ears
- Altered taste, dry mouth.
Tell your doctor as soon as possible if you notice any of the following:
- Muscle tremors, spasms, twitching, jerky movements or sustained muscle contractions
- Abnormal facial movements such as tongue thrusting, repetitive chewing, jaw swinging or grimacing
- A feeling of apathy or not caring about things
- Hallucinations
- Agitation
- Confusion
- Unusually overactive
- Changes in muscle tone, muscle weakness or fatigue
- Numbness or pins and needles
- Problems with breathing, shortness of breath
- Bleeding abnormalities or bruising more easily than normal
- Sensitivity to sunlight.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- Fits or seizures, which may be accompanied by a sudden fever
- Signs of allergy such as rash or hives, swelling of the face, lips, tongue or throat, wheezing, difficulty breathing or swallowing
- Symptoms of sudden fever with sweating, rapid heartbeat and muscle stiffness, which may lead to loss of consciousness
- Palpitations, fainting, shortness of breath, chest pain or irregular heartbeats
- Dark, red or cola coloured urine, muscle weakness and tenderness, stiffness or aching
- Stomach pain, yellowing of the skin, nausea, fever, clammy skin and sweating
- Yellowing of the skin or eyeballs, fever, fatigue, loss of appetite, dark coloured urine or light coloured bowel movements
- A severe skin reactions with painful red areas and large blisters, accompanied by fever and chills, aching muscles and generally feeling unwell
- Symptoms of a high fever, agitation, confusion, trembling and abrupt contractions of muscles
- Signs of an infection such as mouth ulcers, severe chills, fever and sore throat
- Black sticky bowel motions or bloody diarrhoea.
These symptoms are usually rare but may be serious and need urgent medical attention.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell, even if it is not on this list.
After taking ENLAFAX-XR
Storage
Keep your ENLAFAX-XR capsules in their blister pack until it is time to take them. The capsules may not last as well if you take them out of the blister pack.
Keep ENLAFAX-XR capsules in a cool dry place where the temperature stays below 25°C.
Do not store ENLAFAX-XR capsules or any other medicine in the bathroom or near a sink. Do not leave ENLAFAX-XR capsules in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep ENLAFAX-XR capsules and all medication where young children cannot reach it. A locked cupboard, at least one-and-a-half metres above the ground, is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
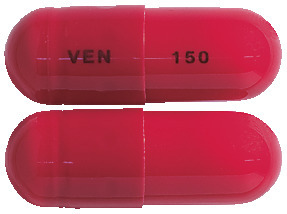
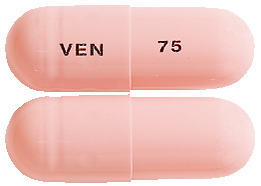
There are two strengths of ENLAFAX-XR capsules, containing 75 mg or 150 mg of venlafaxine (as hydrochloride) in an extended release formulation, which allows for once-a-day dosing.
The 75 mg capsules are flesh opaque-flesh opaque, No 0, capsules printed on cap with VEN, and on the body with 75, containing two white, round, biconvex 37.5 mg film coated tablets and the 150 mg capsules are Scarlet opaque scarlet-opaque, No 00, printed on cap with VEN, and on the body with 150, containing three white, round, biconvex 50 mg film coated tablets.
ENLAFAX-XR 75 mg and 150 mg are available in blister packs containing 28 capsules.
Ingredients
ENLAFAX-XR capsules contain venlafaxine (as hydrochloride) as the active ingredient. ENLAFAX-XR 75 mg and 150 mg capsules contain the following inactive ingredients:
- Ammonio methacrylate copolymer
- Magnesium stearate
- Hypromellose
- Sodium lauryl sulphate
- Basic butylated methacrylate copolymer
- Gelatin
- Titanium dioxide
In addition to these, ENLAFAX-XR 75 mg capsules contain iron oxide red and TekPrint SW-1102 Red Ink. ENLAFAX-XR 150mg capsules contain Indigo carmine, Erythrosine and TekPrint SB-0007P White Ink.
ENLAFAX-XR contains galactose and trace quantities of sulfites.
Supplier
ENLAFAX-XR is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in July 2023.
Australian Registration Numbers:
ENLAFAX-XR venlafaxine (as hydrochloride) 75mg modified release capsules - AUST R 143533
ENLAFAX-XR venlafaxine (as hydrochloride) 150mg modified release capsules - AUST R 143552
ENLAFAX® is a Viatris company trade mark
ENLAFAX XR_cmi\Jul23/00
Published by MIMS August 2023

 Adverse effects are listed in Table 2 in CIOMS frequency categories: common: ≥ 1%; uncommon: ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%.
Adverse effects are listed in Table 2 in CIOMS frequency categories: common: ≥ 1%; uncommon: ≥ 0.1% and < 1%; rare: ≥ 0.01% and < 0.1%; very rare: < 0.01%.



 Molecular formula: C17H27NO2.HCl. Molecular weight: 313.87.
Molecular formula: C17H27NO2.HCl. Molecular weight: 313.87.