What is in this leaflet
This leaflet answers some common questions about Entocort.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Entocort against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What ENTOCORT is used for
Entocort is used to treat Crohn's disease. It can be used to treat acute attacks.
Crohn's disease is an inflammatory disease of the bowel. It mainly affects the small bowel and the first part of the large bowel and causes symptoms such as stomach pain, diarrhoea and fever.
Entocort contains budesonide. This belongs to the group of medicines called corticosteroids, which are used to help reduce inflammation in many parts of the body.
Entocort modified release capsules are designed to release their contents gradually in the small bowel and the first part of the large bowel.
Ask your doctor if you have any questions about why Entocort has been prescribed for you. Your doctor may have prescribed it for another reason.
Entocort is not addictive.
Entocort is only available with a doctor's prescription.
This medicine is not expected to affect your ability to drive a car or operate machinery.
Before you take ENTOCORT
When you must not take it
Do not take Entocort if you have an allergy to:
- any medicine containing budesonide
- any ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- rash, itching or hives on the skin
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body.
Do not give Entocort to children. There is no information available on its use in children.
Do not take this medicine after the expiry date (EXP) printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- any recent infection (including chicken pox and measles)
- tuberculosis
- diabetes
- liver problems
- stomach ulcers
- brittle bones (osteoporosis)
- high blood pressure (hypertension)
- eye problems (such as glaucoma or cataracts).
It may not be safe for you to take Entocort if you have any of these conditions.
Tell your doctor if you have NOT had chicken pox or measles. These diseases may be more serious if you get them while taking Entocort. Your doctor may want to vaccinate you for them before you start on Entocort.
Do not take Entocort if you are pregnant, think you might be pregnant or are breastfeeding unless your doctor says to do so. Ask your doctor about the risks and benefits involved. It is not known if it is safe for you to take Entocort while you are pregnant. It may affect your baby.
Like most corticosteroid medicines, it is recommended that you do not breastfeed while taking Entocort as it is found in breast milk.
If you have not told your doctor about any of the above, tell them before you start taking Entocort.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Entocort may interfere with each other. These include:
- other corticosteroid medicines such as tablets, asthma inhalers, nasal sprays, eye/nose drops
- medicines used to treat fungal infections (e.g. ketoconazole)
- cimetidine, a medicine used to treat reflux and stomach ulcers
- medicines for HIV such as cobicistat.
These medicines may be affected by Entocort or may affect how well it works. You may need different amounts of your medicines, or you may need to use different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Entocort.
Diagnostic tests for pituitary glands activity may show false low values due to suppression of the adrenal function.
How to take ENTOCORT
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
The usual dose is 3 capsules taken once daily in the morning, before breakfast.
How to take it
Swallow Entocort capsules whole with a glass of water.
Do not crush or chew the capsules. If the granules are chewed or crushed they will not work properly.
How long to take it
Continue taking Entocort for as long as your doctor tells you.
A treatment course should normally not exceed 12 weeks.
Entocort should not be stopped suddenly. The dose should be reduced gradually over the last 2-4 weeks.
If you forget to take it
If you miss a dose, take it as soon as you remember, and then go back to taking it as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, pharmacist, the Poisons Information Centre (131 126) or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Entocort. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are using ENTOCORT
Things you must do
Take Entocort exactly as your doctor has prescribed.
If you have not had chicken pox or measles, avoid close contact with anyone who has these diseases while you are taking Entocort. Tell your doctor straight away if you think that you have been exposed to chicken pox or measles.
Tell your doctor if you have an infection while you are taking Entocort. It may not be safe for you to continue taking Entocort if you have an infection.
If you are about to be started on any new medicine, remind your doctor or pharmacist that you are taking Entocort.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking Entocort.
If you become pregnant while using Entocort, tell your doctor or pharmacist immediately.
Things you must not do
Do not eat grapefruit or drink grapefruit juice while you are taking Entocort. Grapefruit juice, but not other fruit juices, can affect Entocort levels in the body. This may increase the chance of getting unwanted side effects.
Do not stop taking Entocort unless your doctor tells you to. If you need to stop taking Entocort, your doctor will tell you how to do it gradually.
Do not use it to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Entocort.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- indigestion, flatulence
- nausea, vomiting, abdominal pain
- rapid heart rate or palpitations
- headache, dizziness
- swelling or rounding of the face, acne, weight gain and bruising more easily
- back pain
- muscle cramps or muscle weakness (may be due to low levels of potassium in blood)
- increased sweating
- tiredness
- trouble sleeping
- tremor, feeling nervous
- mood swings
- depression, anxiety or aggression
- shaking, muscle spasms or twitching
- menstrual problems
- skin rash, itchiness or discolouration.
These side effects are usually mild.
If your medicine has been changed from other oral corticosteroids (e.g. prednisone, prednisolone or methylprednisolone) to Entocort you may notice some symptoms that bothered you earlier, e.g. rash, or pain in muscles and joints. If this happens or you get headaches, nausea or vomiting, or feel tired please contact your doctor.
Tell your doctor or pharmacist if you notice any of the following:
- sign or symptoms of an infection
- blurred vision or other visual disturbances.
These may be serious side effects. You may need medical attention.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- shortness of breath, wheezing or difficulty breathing
- swelling of the face, particularly eyelids, lips, tongue or throat which may cause difficulty in swallowing or breathing
- severe rash.
These are very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some patients.
If you have changed from or used high doses of oral corticosteroids over a long period of time, your adrenal glands may be affected. Your doctor may do tests to check how the adrenal glands are working.
Your doctor may also tell you to take additional oral corticosteroids during periods of stress such as trauma and surgery.
A slowing of the rate of growth maybe observed if Entocort is used in children and adolescents.
Ask your doctor to answer any questions you may have.
After using ENTOCORT
Storage
Keep your Entocort capsules in the bottle until it is time to take them.
Replace the cap firmly after use. If you take Entocort out of the bottle it will not keep well.
Keep Entocort in a cool dry place where the temperature stays below 30°C.
Do not store Entocort or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Entocort or it has passed its expiry date, ask your pharmacist what to do with any capsules you have left over.
Product description
What it looks like
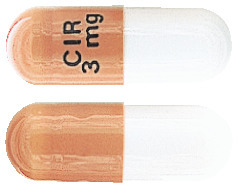
Entocort modified release capsules are two-piece hard gelatin capsules, size 1 with an opaque light grey body and an opaque pink cap. The cap has black print CIR 3mg.
Entocort is supplied in bottles containing 90 capsules.
Ingredients
Each Entocort modified release capsule contains 3 mg of budesonide as the active ingredient.
The capsules also contain the following inactive ingredients:
- ethylcellulose
- tributyl acetylcitrate
- methacrylic acid copolymer
- triethyl citrate
- dimeticone 1000
- polysorbate 80
- purified talc
- Sugar Spheres (ARTG No. 2535).
Each capsule shell is made from gelatin, titanium dioxide, iron oxide black, iron oxide red, iron oxide yellow, colloidal anhydrous silica, liquid paraffin and sodium lauryl sulfate.
The capsules are printed with TekPrint SW-9008 Black Ink (ARTG PI No. 2328).
Supplier
Chiesi Australia Pty Ltd
Suite 3, 22 Gillman Street
Hawthorn East, VIC 3123
Email: [email protected]
Website: www.chiesi.com.au
This leaflet was prepared in January 2023.
Australian Registration Number:
AUST R 62812
Published by MIMS February 2023

 Data from the three controlled clinical trials showed statistically that the relative risk of glucocorticosteroid side effects with Entocort 9 mg is reduced compared to prednisolone 40 mg on an overall basis, and in particular with respect to moon face, acne and buffalo hump. The relative risk for glucocorticoid adverse event, particularly occurrence of moon face, was increased relative to placebo.
Data from the three controlled clinical trials showed statistically that the relative risk of glucocorticosteroid side effects with Entocort 9 mg is reduced compared to prednisolone 40 mg on an overall basis, and in particular with respect to moon face, acne and buffalo hump. The relative risk for glucocorticoid adverse event, particularly occurrence of moon face, was increased relative to placebo.

