SUMMARY CMI
Flufeme®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Flufeme?
Flufeme contains the active ingredient fluconazole. Flufeme capsules is used to treat vaginal thrush, a yeast infection of the vagina.
For more information, see Section 1. Why am I using Flufeme? in the full CMI.
2. What should I know before I use Flufeme?
Do not start treatment if you have ever had an allergic reaction to fluconazole, or any of the ingredients listed at the end of the CMI or any other similar medicines such as miconazole, ketoconazole or clotrimazole.
Tell your doctor if you have had any allergic reaction to any antifungal, or any foods, preservatives or dyes or any other medicines. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
You must not be given Flufeme if you are taking certain medicines. For more information, see Section 2. What should I know before I use Flufeme? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Flufeme and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Flufeme?
- Swallow Flufeme capsules whole with water.
- The treatment for vaginal thrush is one Flufeme capsule. More instructions can be found in Section 4. How do I use Flufeme? in the full CMI.
5. What should I know while using Flufeme?
| Things you should do |
|
| Things you should not do |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Flufeme? in the full CMI.
6. Are there any side effects?
Flufeme is generally well tolerated. Side effects may include nausea, vomiting, abdominal pain, diarrhoea, headache, skin rash or redness, seizures.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
Flufeme®
Active ingredient(s): fluconazole (flu-con-a-zole)
Consumer Medicine Information (CMI)
This leaflet provides important information about using Flufeme. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Flufeme.
Where to find information in this leaflet:
1. Why am I using Flufeme?
2. What should I know before I use Flufeme?
3. What if I am taking other medicines?
4. How do I use Flufeme?
5. What should I know while using Flufeme?
6. Are there any side effects?
7. Product details
1. Why am I using Flufeme?
Flufeme contains the active ingredient fluconazole. Flufeme capsules is used to treat vaginal thrush, a yeast infection of the vagina.
Flufeme belongs to a group of medicines called azole antibiotics.
It works by preventing the growth of the fungal organisms causing the infection in the vagina.
Ask your doctor if you have any questions about why Flufeme has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is not addictive.
2. What should I know before I use Flufeme?
Warnings
Do not start treatment with Flufeme if:
- you are allergic to fluconazole, or any of the ingredients listed at the end of this leaflet, or any medicines related to fluconazole such as miconazole, ketoconazole or clotrimazole. Always check the ingredients to make sure you can use this medicine.
- Symptoms of an allergic reaction include
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- skin rash, itching or hives
You must not be given Flufeme if you are taking any of the following medicines:
- terfinadine or astemizole (a medicine used to treat allergy)
- cisapride (a medicine used to treat stomach problems)
- erythromycin (a medicine used to treat infections)
- pimozide (a medicine used to treat mental illness)
- quinidine (a medicine used to treat irregular heartbeat).
Check with your doctor if you:
- have allergies to any foods, preservatives or dyes or any other medicines
- are taking medicines for any other condition
- have any other medical conditions
- have liver problems
- have heart problems
- have kidney problems
- have thrush more than twice in the last 6 months.
Tell your doctor or pharmacist if you are experiencing any of the following:
- abnormal or irregular vaginal bleeding or blood stained discharge
- vulval or vaginal sores, ulcers or blisters
- lower abdominal pain or burning when passing urine
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Do not take this medicine if you are pregnant or suspect you are pregnant.
It may affect your developing baby if you take it during pregnancy.
Do not take this medicine if you are breastfeeding.
The active ingredient in Flufeme passes into breast milk and there is a possibility that your baby may be affected.
Talk to your doctor about the need for an additional method of contraception while being given Flufeme.
Flufeme may decrease the effectiveness of some birth control pills.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines should not be taken with Flufeme.
These are listed under Section 2. What should I know before treatment with Flufeme.
Some medicines and Flufeme may interfere with each other. These medicines and some others may be affected by Flufeme and affect how it works. You may need different amounts of your medicines, or you may need to take different medicines. These include:
- some medicines for diabetes such as glipizide, tolbutamide or glibenclamide
- some antibiotics, antiviral and antifungal drugs such as rifampicin, rifabutin, zidovudine, amphotericin B, azithromycin, saquinavir or voriconazole
- some drugs used for heart problems, such as amiodarone or verapamil
- some drugs used in problems with the immune system, such as ciclosporin, tacrolimus, sirolimus or tofacitinib
- some medicines used to lower cholesterol, such as atorvastatin, simvastatin or fluvastatin
- cyclophosphamide, vincristine, vinblastine, olaparib or ibrutinib (used to treat certain types of cancers)
- tolvaptan (used to treat low levels of sodium in your blood or for kidney problems)
- halofantrine (used to treat malaria)
- warfarin (used to stop blood clots)
- phenytoin (used to treat epilepsy)
- prednisone (used to treat inflammation or suppress the immune system)
- theophylline (used to treat asthma)
- some benzodiazepines such as midazolam
- lemborexant (used to treat insomnia or sleeping difficulties)
- ivacaftor (used to manage cystic fibrosis)
- lurasidone (used to manage schizophrenia)
- hydrochlorothiazide (used for treating fluid problems)
- the contraceptive pill (birth control pill)
- carbamazepine (used in the treatment of epilepsy and bipolar disorder)
- NSAIDS such as naproxen, diclofenac and celecoxib
- Vitamin A
- opioid pain killers such as alfentanil, fentanyl and methadone
- losartan (used for treating high blood pressure)
- antidepressants such as amitriptyline and nortriptyline.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Flufeme.
4. How do I use Flufeme?
How much to take
- The treatment for thrush is one Flufeme capsule.
When to take Flufeme
- This medicine can be taken before, with or after food and can be taken at any time of the day.
How to take Flufeme
- Swallow the capsules whole with water.
If you use too much Flufeme
If you think that you have used too much Flufeme, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Flufeme?
Things you should do
- Tell your doctor or pharmacist if symptoms of your infection do not improve within 3 days or if they become worse.
- Things that may help you avoid thrush in the future:
- after urinating, the toilet tissue should be used as a blotter, rather than used with a forward or backward motion. Similarly, to avoid the possibility of spreading organisms from the rectum to the vaginal tract after a bowel movement, a wiping motion away from the vagina should be used when applying toilet tissue.
- underwear, night attire, towels and linen should be changed daily
- wear cotton briefs, stockings and loose-fitting clothing rather than tight synthetic clothing
- wash regularly but do not wash and dry yourself harshly
- avoid perfumed soaps, bath additives and vaginal deodorants. - Remind any doctor, dentist, or pharmacist you visit that you are using Flufeme.
Things you should not do
- Do not start treatment if you have ever had an allergic reaction to any medicine containing fluconazole, any of the ingredients listed at the end of this CMI
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not use Flufeme to treat any other medical complaints unless your doctor tells you to.
Driving or using machines
Be careful when driving vehicles or operating machinery as occasional dizziness or seizures may occur.
Drinking alcohol
No information available.
Looking after your medicine
- Keep your medicine in its original pack until it is time to take it.
- If you take it our of the pack it may not keep well.
Store it in a cool dry place below 25°C away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Gut or Gastrointestinal related
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Allergy or reaction related
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Flufeme contains
| Active ingredient (main ingredient) | fluconazole |
| Other ingredients (inactive ingredients) |
|
| Potential allergens |
|
Do not take this medicine if you are allergic to any of these ingredients.
What Flufeme looks like
Flufeme 150 mg - capsules with a white cap and white body, printed with FC150 (AUST R 132827).
Available in blisters of 1 capsule.
Who distributes Flufeme
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park, NSW 2113
Tel: 1800 726 369
Novartis New Zealand Ltd
PO Box 99102
Newmarket, Auckland 1149
New Zealand
Tel: 0800 354 335
This leaflet was revised in October 2022.
Published by MIMS December 2022

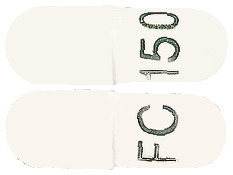

 Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported in association with fluconazole treatment (see Section 4.4 Special Warnings and Precautions for Use).
Drug reaction with eosinophilia and systemic symptoms (DRESS) has been reported in association with fluconazole treatment (see Section 4.4 Special Warnings and Precautions for Use).

