What is in this leaflet
This leaflet answers some common questions about FLUTAMIN.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking FLUTAMIN against the benefits this medicine is expected to have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may want to read it again.
What FLUTAMIN is used for
FLUTAMIN is used in combination with medical castration to treat prostate cancer.
The Prostate Gland
The prostate gland is a walnut-sized gland that surrounds the upper part of the urethra. The urethra is a tube through which urine and sperm exit through the tip of the penis.
The main job of the prostate gland is to produce fluid that nourishes and transports sperm. The gland is vulnerable to two common but unrelated medical problems - enlargement and cancer.
Cancer of the Prostate
Prostate cancer is the most common cancer among Australian men. About 1 in every 10 men will develop the disease in his lifetime. The causes of prostate cancer are unknown but the disease is often responsive to treatment.
Who is at Risk?
Prostate cancer is more common in older men; 80% of men who develop prostate cancer are diagnosed when they are over the age of 65. However, some men develop the disease when they are younger.
What is Maximal Androgen Blockade?
Prostate cancer cells need androgens (male hormones) to grow. There are two sources of androgens in your body: the testes and the adrenal glands (small glands that sit on top of your kidneys). Maximal androgen blockade which combines castration and a medicine called an antiandrogen, is designed to prevent the androgens from both sources reaching and nourishing the cancer cells.
Castration stops the production of testosterone by the testes. Castration can be accomplished either surgically or medically. Surgical castration, called an orchidectomy, is the actual removal of the testes. Medical castration involves taking hormonal drugs known as luteinising hormone-releasing hormone (LHRH) agonists which stop the testes from producing testosterone. Two examples of LHRH agonists are Lucrin (leuprolide) and Zoladex (goserelin acetate). LHRH agonists are given as monthly injections.
While castration "shuts off" the production of testosterone by the testes, it does nothing to stop the androgens produced by the adrenal glands from continuing to nourish the cancer cells.
How FLUTAMIN Works
To block the adrenal androgens from reaching the cancer cells, your doctor has prescribed FLUTAMIN, an antiandrogen. FLUTAMIN is used in combination with medical castration. The combination of medical castration and FLUTAMIN, blocks androgens from all sources; this is called maximal androgen blockade.
Ask your doctor if you have any questions about why FLUTAMIN has been prescribed for you. Your doctor, however, may prescribe FLUTAMIN for another reason.
FLUTAMIN is available only with a doctor's prescription.
There is no evidence that FLUTAMIN is addictive.
Before you take FLUTAMIN
When you must not take it
Do not take FLUTAMIN if you are allergic to medicines containing flutamide or any of the ingredients listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing; wheezing or shortness of breath.
Do not take FLUTAMIN if you have serious liver problems.
Do not take FLUTAMIN after the expiry date (EXP) printed on the pack.
Do not take FLUTAMIN if the packaging is torn or shows signs of tampering.
FLUTAMIN is for use by men only. It should not be taken by women who are pregnant or breastfeeding.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
You must tell your doctor if:
- you are allergic to any other medicines, or any foods, dyes or preservatives
- you have any other medical conditions or health problems
- you are pregnant or breastfeeding.
- you have liver problems
- you have or have had heart disease, a condition called 'long QT syndrome' or a family history of this heart condition
- you have diabetes
- you have or have had a history of anaemia, a low red blood cell count
- you have low bone mineral density (BMD)
- you have osteoporosis or a strong family history of osteoporosis or bone fracture
- you consume large quantities of alcohol and/or tobacco
- you take medicines to treat epilepsy/fits
- you take medicines to reduce the activity of your immune system.
If you have not told your doctor about any of the above, tell him/her before you start taking FLUTAMIN.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you buy without a prescription from a pharmacy, supermarket or health food shop. There may be some interference between FLUTAMIN and some other medicines, including:
- Oral anticoagulants such as warfarin, a medicine used to prevent blood clots
- Theophylline, used for asthma
- Medicines used to treat irregular heart rhythm e.g. quinidine, disopyramide, amiodarone, sotalol, dofetilide, ibutilide, dronedarone, flecainide, propafenone
- Medicines that help correct chemical imbalances in the brain which may cause mental illness or behavioural disturbances e.g. chlorpromazine,
- Medicines used to treat depression e.g. amitriptyline, nortriptyline
- Medicines used to help control pain e.g. methadone
- Medicines which treat infection caused by bacteria e.g. erythromycin, clarithromycin, azithromycin, moxifloxacin
- Quinine, an antimalarial
- Azole antifungals which prevent growth of fungal and yeast organisms
- Medicines that help stop nausea and vomiting e.g. ondansetron
- Medicines used in the treatment of asthma e.g. salbutamol.
Your doctor can tell you what to do if you are taking this medicine.
If you are not sure whether you are taking the above medicine, check with your doctor or pharmacist. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking FLUTAMIN.
How to take FLUTAMIN
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist.
How much to take
Take one tablet by mouth three times a day, exactly as directed by your doctor.
When to take it
Taking FLUTAMIN every eight hours is the best way of making sure the therapy will block the androgens in your body.
Take one tablet when you first get up, one in the afternoon and one at bedtime.
FLUTAMIN can be taken with or without food.
Do not use meals as a reminder to take FLUTAMIN because meals are not normally 8 hours apart.
How to take it
Swallow the tablets whole with a full glass of water.
How long to take it for
Continue to take FLUTAMIN regularly, for as long as your doctor tells you to.
Do not stop taking this medicine without first checking with your doctor.
If you forget to take it
If you forget to take it, ask your doctor or pharmacist for advice.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you have trouble remembering when to take your medicine, ask your pharmacist for advice.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much FLUTAMIN.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
While you are taking FLUTAMIN
Things you must do
Tell your doctor or pharmacist if you are taking any other medicines. Your doctor may want you to have your blood tested occasionally during your therapy.
Take FLUTAMIN exactly as your doctor has prescribed.
Tell all doctors, dentists and pharmacists who are treating you that you are taking FLUTAMIN.
Tell your doctor or pharmacist that you are taking FLUTAMIN if you are about to be started on any new medicines.
Things you must not do
Do not stop taking FLUTAMIN, or change the dose, without checking with your doctor.
Do not use FLUTAMIN to treat any other complaints unless your doctor tells you to.
Do not give FLUTAMIN to anyone else, even if their symptoms seem similar to yours.
Things to be careful of
FLUTAMIN generally does not cause any problems with your ability to drive a car or operate machinery. However, make sure you know how you react to FLUTAMIN before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy or light-headed.
Side Effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking FLUTAMIN.
FLUTAMIN helps most people with prostate cancer, but it may have unwanted side effects in some people.
All medicines have side effects. Sometimes they are serious, most of the time they are not. Although not all of these side effects may occur, if they do occur they may need medical attention.
Ask your doctor or pharmacist any questions you may have.
Do not be alarmed by this list of side effects. You may not experience any of them.
Tell your doctor immediately if you notice any of the following symptoms. These may indicate liver disorder, which has been reported very rarely with FLUTAMIN.
- itching of the skin
- dark urine (amber or yellow-green urine is not a cause for concern)
- nausea, vomiting
- persistent lack of appetite
- yellow eyes or skin
- tenderness in the right upper stomach
- constant tiredness or "flu-like" symptoms.
Patients receiving the combination of FLUTAMIN plus medical castration may have:
- diarrhoea
- hot flushes (sudden sweating and feelings of warmth)
- increased breast size
- nausea (feeling sick)
- vomiting
Unwanted effects that may occur rarely include:
- bleeding or bruising more than normal
- loss of appetite
- injection site irritation or rash
- swelling of feet or lower legs
- shortness of breath
- muscle ache or twitching
- high blood pressure
- liver damage or brain coma from liver illness
- yellow discolouration of the skin or eyes due to failure to remove bile
Unwanted effects that may occur very rarely include:
- difficult or labored breathing
- feeling fatigued
- excessive thirst and urination
- unusual weight loss or weight gain
- nausea and vomiting
- slow healing cuts or infection
- blurred vision
- drowsiness
- confusion
- depression
- anxiety
- nervousness
- decrease in sexual desire or sexual ability
- difficulty sleeping
- always feeling hungry
- headaches and feeling dizzy
- mood swings
- leg cramps, tingling/numbness in hands and/or feet
- heart rhythm disorder
Tell your doctor or pharmacist if any of these occur.
In general, patients will experience few additional effects when FLUTAMIN therapy is added to medical castration.
The following tips may help if you are experiencing diarrhoea:
- drink plenty of fluids
- cut down on dairy products
- try eating smaller food portions
- eat more carbohydrates
- in consultation with your doctor, you may also try taking medication to manage diarrhoea.
Since a variety of medications can cause diarrhoea, it is important to tell your physician about all the medicines you take, including those that are non-prescription.
Any therapy that stops the production of male hormones, such as medical or surgical castration, will affect your sex drive. It is important to remember that the reduction of androgen levels may make erection more difficult. If you experience problems, please ask your doctor about available treatment options.
Check with your doctor as soon as possible if you have any problems while taking FLUTAMIN even if you do not think the problems are connected with the medicine or are not listed in this leaflet.
This is not a complete list of all possible side effects.
Other side effects not listed above may also occur in some people. If you notice any other side effects, check with your doctor.
Do not be alarmed by this list of possible side effects. You may not experience any of them
After using FLUTAMIN
Storage
Keep FLUTAMIN where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they will not keep well.
Keep FLUTAMIN in a cool dry place, away from direct light where the temperature stays below 30°C.
Do not store FLUTAMIN or any other medicine in the bathroom or near a sink.
Do not leave FLUTAMIN in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking FLUTAMIN, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
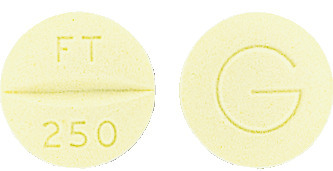
FLUTAMIN is a biconvex, round, yellow tablet debossed "FT" breakline "250" on one side and "G" on the reverse.
FLUTAMIN is available in packs of 100 tablets.
Ingredients
The active ingredient in FLUTAMIN is flutamide.
Each FLUTAMIN tablet contains 250 mg of flutamide.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- microcrystalline cellulose
- maize starch
- pregelatinised maize starch
- sodium lauryl sulfate
- colloidal anhydrous silica
- magnesium stearate.
FLUTAMIN contains sulfites and sugars as lactose.
Supplier
FLUTAMIN is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in November 2023.
Australian registration number:
AUST R 65675
FLUTAMIN_cmi\Nov23/00
Published by MIMS March 2024

 Chemical name: 2-methyl-N-[4-nitro-3- (trifluoromethyl)- phenyl]propanamide.
Chemical name: 2-methyl-N-[4-nitro-3- (trifluoromethyl)- phenyl]propanamide.