SUMMARY CMI
GILENYA®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
▼ This medicine is new or being used differently. Please report side effects. See the full CMI for further details.
1. Why am I using Gilenya?
Gilenya contains the active ingredient fingolimod hydrochloride. Gilenya is used to treat relapsing forms of multiple sclerosis in adults, children and adolescents (10 years of age and above). For more information, see Section 1. Why am I using Gilenya? in the full CMI.
2. What should I know before I use Gilenya?
Do not use if you have ever had an allergic reaction to fingolimod hydrochloride or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use Gilenya? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Gilenya and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Gilenya?
Adults: The usual dose is one 0.5 mg capsule taken once a day. Children and adolescents: The dose is dependent on body weight. More instructions can be found in Section 4. How do I use Gilenya? in the full CMI.
5. What should I know while using Gilenya?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Gilenya? in the full CMI.
6. Are there any side effects?
Common side effects: flu symptoms, headache, diarrhoea. Serious side effects: coughing with phlegm, chest pain, shingles/ herpes zoster, slow or irregular heartbeat, blurred vision, skin nodules. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
FULL CMI
GILENYA®
Active ingredient(s): fingolimod hydrochloride
Consumer Medicine Information (CMI)
This leaflet provides important information about using Gilenya. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Gilenya.
Where to find information in this leaflet:
1. Why am I using Gilenya?
2. What should I know before I use Gilenya?
3. What if I am taking other medicines?
4. How do I use Gilenya?
5. What should I know while using Gilenya?
6. Are there any side effects?
7. Product details
1. Why am I using Gilenya?
Gilenya contains the active ingredient fingolimod. Gilenya belongs to a group of medicines known as sphingosine 1-phosphate (S1-P) receptor modulators.
Gilenya is used to treat adults, children and adolescents (10 years of age and above) with relapsing forms of multiple sclerosis (MS).
This medicine can alter the way the body's immune system works and slows the progression of physical disability and decreases the number of flare-ups (relapses) in patients with relapsing MS.
MS is a long-term condition that affects the central nervous system (CNS), particularly how the brain and spinal cord work. In MS, inflammation destroys the protective cover around the nerves (called myelin) and stops the nerves from working properly.
The cause of MS is unknown, but it is thought that an abnormal response by the body's immune system plays an important part in the process which damages the CNS.
Gilenya helps to fight against attacks on myelin by the immune system and by stopping the cells that cause inflammation from reaching the brain. This reduces nerve damage caused by MS.
Gilenya may also have a direct and beneficial effect on certain brain cells (neural cells) involved in repairing or slowing down the damage of MS.
Ask your doctor if you have any questions about how Gilenya works or why this medicine has been prescribed for you or your child.
2. What should I know before I use Gilenya?
Warnings
Do not use Gilenya if:
- you are allergic to fingolimod, or any other similar medicines (such as medicines of the same class or with a similar structure), or any of the ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin. - you have had a heart attack, unstable angina, stroke or warning stroke or certain types of heart failure in the last 6 months
- have certain types of irregular or abnormal heartbeat (arrhythmia)
- you are taking or have recently taken medicine for irregular heartbeat such as quinidine, disopyramide, amiodarone or sotalol (due to a possible added effect on irregular heartbeat).
Check with your doctor if you:
- have had heart problems, a stroke or warning of a stroke
Checking the health of your heart is important. If any of the following applies to you, your doctor may decide not to use Gilenya or may refer you to a cardiologist for further advice before commencing your first dose of Gilenya:
- irregular or abnormal heartbeat
- severe heart disease
- uncontrolled high blood pressure
- history of stroke or other diseases related to blood vessels in the brain
- severe breathing difficulties when asleep (sleep apnoea that is not treated)
- a heart rhythm disturbances (called QTc prolongation or abnormal ECG heart tracing) or the risk of these disturbances
- slow heart rate or if you have a history of sudden loss of consciousness (fainting).
Your doctor will test your status of the antibody against this virus and may decide to vaccinate you (if you do not have antibodies to this virus). In this case you will start Gilenya treatment one month after the full course of the vaccination is completed.
Children or adolescents (10 years of age and above) need to have completed their vaccination schedule before starting treatment with Gilenya.
- plan to receive a vaccine
You should not receive certain types of vaccines (called "live attenuated vaccines") during and up to 2 months after treatment with Gilenya (see Section 3 "What if I am taking other medicines?"). - have any other medical conditions:
- a lowered immune response (due to a disease or medicines that suppress the immune system). See "taking other medicines". You may get infections more easily or an infection you already have may get worse.
- problems with your liver. Gilenya may affect your liver function. - have an infection as it may get worse
Infections can be serious and sometimes life-threatening. Before you start taking Gilenya, your doctor will confirm whether you have enough white blood cells (these fight infections) in your blood. - take any medicines for any other condition
If you are not sure whether any of the above conditions apply to you, your doctor can advise you.
Before you start treatment, you will have:
- a blood test to check your liver function before and during treatment with Gilenya and until two months after stopping treatment. If liver problems are detected your doctor may decide to discontinue treatment.
- a skin examination is recommended before you start and at regular intervals during treatment. Your doctor will decide what to do if skin problems are noticed.
- an eye examination before you start treatment and at regular intervals afterwards is recommended if you have or have had one of the following conditions:
- visual disturbances or other signs of swelling in the central vision area at the back of the eye (a condition known as macular oedema)
- inflammation or infection of the eye (uveitis)
- diabetes - vaccination against human papilloma virus (HPV) is recommended. If you are female, your doctor will also recommend HPV screening.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
If you are female, a pregnancy test is recommended before starting treatment to check if you are pregnant.
Check with your doctor if you are pregnant or intend to become pregnant.
You should avoid becoming pregnant while taking Gilenya or in the two months after you stop taking it because Gilenya may harm your unborn baby.
If you become pregnant while taking Gilenya, tell your doctor without delay.
You and your doctor will decide what is best for you and your baby.
You should not breast-feed while you are taking Gilenya.
Gilenya can pass into breast milk and there is a risk of serious side effects for a breast-fed baby.
Elderly
Experience with Gilenya in older people (more than 65 years old) is limited.
Children under 10 years
Gilenya has not been studied in children under 10 years of age.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Gilenya and affect how it works. These include:
- medicines for an irregular or abnormal heartbeat such as quinidine, procainamide, amiodarone or sotalol.
- medicines that slow down heartbeat such as atenolol (a beta-blocker); verapamil, diltiazem or (calcium channel blockers) or ivabradine or digoxin. Your doctor may decide not to use Gilenya or may refer you first to a cardiologist to switch to medicines that do not slow your heart rate or to decide how you should be observed after the first dose of Gilenya
- medicines that can cause an abnormal heart rhythm called Torsades de Pointes such as citalopram, chlorpromazine, haloperidol, methadone or erythromycin
- medicines that suppress or modulate the immune system including other medicines used to treat MS such as beta-interferon, glatiramer acetate, natalizumab, mitozantrone, dimethyl fumarate, teriflunomide, alemtuzumab or corticosteroids due to a possible added effect on the immune system
- vaccines. If you need to receive a vaccine, seek your doctor's advice first. During and up to 2 months after treatment with Gilenya, administration of some vaccines containing live virus (live attenuated vaccines) may result in an infection that the vaccination is designed to prevent, while others may not work as well.
You may need to take different amounts of your medicines or to take different medicines while you are taking Gilenya.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Gilenya.
4. How do I use Gilenya?
First dose precaution
Because Gilenya may have a short-term effect on your heart rate when you take the first dose (or when children/adolescents switch from the 0.25 mg capsule to the 0.5 mg capsule), you will be required to have the health of your heart checked:
- Before your first dose of Gilenya
- 6 hours after taking your first dose of Gilenya, and
- if you start Gilenya again after a break from therapy (depending on how long the break is and how long you have been receiving Gilenya treatment).
You will need to stay at the doctor's office or clinic for 6 hours after taking the first dose of Gilenya (or after taking the first dose of 0.5 mg when your child switches from the 0.25 mg capsule daily dose) so that your heart rate and blood pressure can be checked each hour. Your doctor will also check and record the electrical activity of your heart (using a test called an ECG) and check your heart rhythm.
Tell your doctor if you feel dizzy, tired, or are conscious of your heartbeat.
At the end of the 6-hour observation period, you will be required to have a second ECG.
In case of unusual ECG or slow heart rate at the end of the 6-hour observation period, you may be observed for longer and overnight if necessary. In this case, the same observation process that took place for your first dose of Gilenya will also apply for your second dose.
At the beginning of treatment, Gilenya can cause the heart rate to slow down in some patients. If your heart rate slows down after your first dose, you may feel dizzy or tired or be consciously aware of your heartbeat. If your heart rate slows down too much or your blood pressure drops, you may need treatment without delay. Slow heart rate usually returns to normal within one month.
Gilenya can also cause an irregular heartbeat in some patients, especially after the first dose. Irregular heartbeat usually returns to normal in less than one day.
How much to take
Adults
- The usual dose is one capsule per day (0.5 mg of fingolimod).
Children and adolescents (10 years of age and above)
- The dose depends on the body weight:
- Children and adolescents who weigh 40 kg or less: one 0.25 mg capsule per day.
- Children and adolescents with a body weight above 40 kg: one 0.5 mg capsule per day.
- Children and adolescents who started on one 0.25 mg capsule per day and reach a stable body weight above 40 kg will be instructed by their doctor to switch to one 0.5 mg capsule per day. In this case, it is recommended to repeat the first dose observation period.
Do not exceed the recommended dose.
When to take Gilenya
- Gilenya should be taken at about the same time each day.
- Taking it at the same time each day will have the best effect. It will also help you remember when to take it. It does not matter if you take this medicine before or after food.
How to take Gilenya
- Swallow the Gilenya capsule with a glass of water.
- Gilenya can be taken with or without food.
How long to take Gilenya
- Continue taking your medicine for as long as your doctor tells you to.
Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue. - Do not stop taking Gilenya unless your doctor tells you to.
Your symptoms may return or become worse if you stop the treatment. Tell your doctor if you have worsening of MS symptoms after stopping Gilenya.
Gilenya will stay in your body for up to 2 months after you stop taking it. Your white blood cell count (lymphocyte count) may also remain low during this time and the side effects described in this leaflet may still occur.
- If you stop taking Gilenya:
- for 1 day or more during the first 2 weeks of treatment, or
- for more than 7 days during weeks 3 and 4 of treatment, or
- for more than 2 weeks after your first month of Gilenya treatment, the initial effect on your heart rate may occur again.
If you restart Gilenya therapy after a break, your doctor may decide to monitor your heart rate and blood pressure every hour, to run ECGs, or to monitor you overnight.
After using Gilenya
- Tell your doctor straight away, if you believe your MS is getting worse after you have stopped treatment with Gilenya, because it could be serious.
- Symptoms of MS can return and may become worse compared to before or during treatment.
If you forget to use Gilenya
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
Your doctor may decide to observe you at the time you take the next dose.
This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you use too much Gilenya
If you think that you have used too much Gilenya or taken a first dose of Gilenya by mistake, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Your doctor may decide to observe you with hourly heart rate and blood pressure measurements, run ECGs, and he/she may decide to monitor you overnight.
Symptoms of an overdose may include:
- swelling in hands or feet
- tingling or numbness in hands or feet
- muscle pain
- fever.
5. What should I know while using Gilenya?
Things you should do
- Avoid becoming pregnant while taking Gilenya or in the two months after you stop taking it because Gilenya may harm your unborn baby.
- Talk to your doctor about the associated risk. Talk with your doctor about reliable methods of birth control that you should use during treatment and for 2 months after you stop treatment. - Limit your exposure to the sun and UV rays by wearing appropriate protective clothing and regularly applying sunscreen with a high degree of UV protection.
This will help minimise your risk of developing skin cancers. - Keep all of your doctor's appointments so that your progress can be checked.
Your doctor will do regular checks to help prevent you from having side effects from the medicine. This includes blood tests to check your liver function and regular skin checks. - If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Gilenya.
Remind any doctor, dentist or pharmacist you visit that you are using Gilenya.
Call your doctor straight away if you:
- become pregnant while taking this medicine.
Gilenya should not be taken if you are pregnant. - think you have an infection, fever or feel like you have the flu.
You may get infections more easily while you are taking Gilenya and for up to 2 months after you stop taking it. Any infection that you already have may get worse. Infections can be serious and sometimes life-threatening. - notice any changes in your vision, especially if:
- the centre of your vision gets blurry or has shadows
- if you develop a blind spot in the centre of your vision
- if you have problems seeing colours or fine detail.
Gilenya may cause macular oedema uncommonly (swelling of a small area at the back of the eye). When this side effect does occur, it usually happens in the first 4 months of treatment. Your chance of developing macular oedema is higher if you have diabetes or have had an inflammation of the eye called uveitis. It can cause some of the same vision symptoms as an MS attack (optic neuritis).
- notice any skin nodules (e.g., shiny pearly nodules), patches or open sores that do not heal within weeks.
Skin cancers have been reported in MS patients treated with Gilenya. Symptoms may include abnormal growth or changes of skin tissue (e.g., unusual moles) which may change in colour, shape or size over time. Your doctor should carry out regular skin examinations during your treatment with Gilenya. - notice any of following symptoms while you are taking Gilenya because it could be serious:
- signs that your MS is getting worse (e.g., weakness or visual change) or if you notice any new or unusual symptoms. These may be the symptoms of a rare brain disorder caused by infection, called progressive multifocal leukoencephalopathy (PML) or a condition called tumefactive lesions. Your doctor may organise an MRI scan to decide if you need to stop taking Gilenya.
- If you think you have an infection; a fever; feel like you have the flu, or have a headache accompanied by stiff neck, sensitivity to light, nausea, and/or confusion, or seizures/fits (these may be symptoms of meningitis and/or encephalitis)
- sudden onset of severe headache, confusion, seizures and vision changes which are symptoms of a condition called posterior reversible encephalopathy (PRES)
- swelling in your neck, armpits or groin, persistent tiredness, fever, night sweats, shortness of breath, unexplained weight loss, itchy skin which are symptoms of lymphoma
- unexplained nausea, vomiting, abdominal pain, tiredness, yellowing of the skin or whites of your eyes, abnormally dark urine. These may be signs of liver injury.
Things you should not do
- You should not receive certain types of vaccines (live attenuated vaccines) during and for up to 2 months after treatment with this medicine
- Do not give this medicine to anyone else, even if their condition seems similar to yours.
- Do not use it to treat any other complaints unless your doctor tells you to.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Gilenya affects you.
Gilenya may cause dizziness in some people.
Your doctor will tell you whether your illness allows you to drive vehicles and use machines safely.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep your medicine in the original container until it is time to take it.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
General
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Lungs related
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Some side effects may not give you any symptoms and can only be found when tests are done. These include:
- hypertension (increase in blood pressure)
- higher levels of liver enzymes and/or liver injury
- increased level of blood fat (triglycerides)
- changes to blood cell counts
- abnormal lung function test results.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Gilenya contains
| Active ingredient (main ingredient) | fingolimod |
| Other ingredients (inactive ingredients) | 0.5 mg capsule:
0.25 mg capsule:
|
| Potential allergens |
|
Do not take this medicine if you are allergic to any of these ingredients.
What Gilenya looks like
Gilenya 0.25mg hard capsules - ivory opaque body and cap, with black radial imprint “FTY 0.25mg” on cap and black radial band on body, contains white to almost white powder (Aust R 300029).
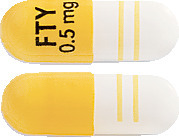
GILENYA 0.5mg hard capsules - white opaque body and bright yellow opaque cap; radial imprint with black ink, "FTY 0.5 mg" on cap and two radial bands imprinted on the body with yellow ink, containing white to almost white powder (Aust R 169890).
Gilenya capsules are available in packs containing 28 capsules.
Who distributes Gilenya
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1 800 671 203
This leaflet was prepared in August 2023.
(gil090221c_v2 based on PI gil090221i)
Published by MIMS October 2023





 Study D2302 (TRANSFORMS) was a 1 year randomized, double blind, double dummy, active (interferon beta-1a 30 micrograms, intramuscular, once weekly) controlled phase III study in patients with RRMS who had not received any natalizumab in the previous 6 months. Prior therapy with interferon-beta or glatiramer acetate up to the time of randomization was permitted.
Study D2302 (TRANSFORMS) was a 1 year randomized, double blind, double dummy, active (interferon beta-1a 30 micrograms, intramuscular, once weekly) controlled phase III study in patients with RRMS who had not received any natalizumab in the previous 6 months. Prior therapy with interferon-beta or glatiramer acetate up to the time of randomization was permitted.
 Pooled results of studies D2301 and D2302 showed a consistent reduction of annualised relapse rate compared to comparator in subgroups defined by gender, age, prior multiple sclerosis therapy, disease activity or disability levels at baseline.
Pooled results of studies D2301 and D2302 showed a consistent reduction of annualised relapse rate compared to comparator in subgroups defined by gender, age, prior multiple sclerosis therapy, disease activity or disability levels at baseline.



