SUMMARY CMI
IBAVYR®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using IBAVYR?
IBAVYR contains the active ingredient ribavirin. IBAVYR is used in combination with other oral agents for the treatment of chronic hepatitis C (CHC). CHC is a viral infection of the liver. For more information, see Section 1. Why am I using IBAVYR? in the full CMI.
2. What should I know before I use IBAVYR?
Do not use if you have ever had an allergic reaction to ribavirin or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use IBAVYR? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with IBAVYR and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use IBAVYR?
- Take IBAVYR exactly as your doctor has directed.
More instructions can be found in Section 4. How do I use IBAVYR? in the full CMI.
5. What should I know while using IBAVYR?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using IBAVYR? in the full CMI.
6. Are there any side effects?
Mild side effects include headache, fatigue, fever and chills, weakness, sleeplessness, stomach pain, discomfort or constipation, muscular ache and pain or joint pain, agitation, irritability, mood swings, or disturbance in attention, hair loss/change in hair texture, itching, rash or dry or redness of the skin, sore throat, cough, shortness of breath back pain. Serious side effects include signs of anaemia such as tiredness, being short of breath and looking pale, signs of liver decompensation such as a swollen abdomen lower back or side pain, severe stomach pain, fever or chills beginning after a few weeks of treatment, persistent cough or shortness of breath, depression, confusion, trouble sleeping, thinking or concentrating and allergic reactions.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
IBAVYR®
Active ingredient: Ribavirin
Consumer Medicine Information (CMI)
This leaflet provides important information about using IBAVYR. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using IBAVYR.
Where to find information in this leaflet:
1. Why am I using IBAVYR?
2. What should I know before I use IBAVYR?
3. What if I am taking other medicines?
4. How do I use IBAVYR?
5. What should I know while using IBAVYR?
6. Are there any side effects?
7. Product details
1. Why am I using IBAVYR?
IBAVYR contains the active ingredient ribavirin. Ribavirin belongs to a group of medicines called antivirals.
IBAVYR is used in combination with other oral agents for the treatment of chronic hepatitis C (CHC).
CHC is a viral infection of the liver. There are different types of the hepatitis C virus, referred to as genotypes. If this viral infection is not managed in some people, the liver becomes badly damaged and scarred. This is called cirrhosis. Cirrhosis can cause the liver to stop working. CHC may also be associated with the development of hepatocellular carcinoma.
IBAVYR is not effective when used alone and must only be used in combination with other oral agents. For more information please refer to the respective Consumer Medicine Information of the other oral agent used during the treatment.
2. What should I know before I use IBAVYR?
Warnings
Do not use IBAVYR if:
- you are allergic to ribavirin, or any of the ingredients listed at the end of this leaflet, or any other similar medicines
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
- you are taking didanosine (a medicine used to treat patients with human immunodeficiency virus (HIV).
- you have blood disorders including anaemia (low number of red blood cells), thalassaemia (Mediterranean anaemia) or sickle-cell anaemia
If you are not sure whether you should start taking IBAVYR, talk to your doctor or pharmacist.
Check with your doctor if you:
- have any other medical conditions
- have, or have ever had any of the following medical conditions before you start taking IBAVYR:
- heart problems such as congestive heart failure, irregular or very fast heartbeat, heart disease, or you have ever had a heart attack
- anaemia (a low number of red blood cells)
- kidney problems
- liver problems other than hepatitis C
- organ transplant
- HIV
- Also refer to the respective Consumer Medicines Information of the other oral agent used during the treatment, for other things to be careful of when IBAVYR is taken in combination with other oral agents.
- take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Do not use IBAVYR if you or your partner is pregnant or planning to become pregnant.
IBAVYR may cause birth defects and/or death of an unborn baby. It is very important that you or your partner avoid becoming pregnant during treatment and for 6 months after treatment. This is because IBAVYR Tablets can affect the sperm as well as the unborn child.
If you are or your partner is a woman of childbearing age, you or your partner must have a negative pregnancy test before treatment with IBAVYR Tablets starts.
You or your partner must also have a negative pregnancy test each month during treatment and for the 6 months after treatment is stopped. Two effective forms of contraception must be used, one by each partner, male and female, during treatment with IBAVYR Tablets and for the 6 months after treatment is completed. IBAVYR Tablets can cause harm to the unborn child if a pregnant woman takes IBAVYR Tablets herself during pregnancy or has unprotected sex (sex without using a condom) with a man who is taking IBAVYR Tablets. IBAVYR Tablets can damage the sperm and the embryo (unborn child).
Do not use IBAVYR Tablets if you are breastfeeding.
It is not known if IBAVYR passes into breast milk. Therefore to avoid any potential side effects in the nursing infant, nursing mothers should stop breast feeding when taking IBAVYR.
Use in children and adolescents
Do not give IBAVYR to children under 18 years of age
Safety and effectiveness in children has not been established.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
You must not take IBAVYR if you are taking didanosine (a medicine used to treat HIV).
You must tell your doctor if you are taking:
- stavudine, zidovudine or lamivudine (medicines used to treat HIV),
- azathioprine (a medicine used to treat organ transplant patients),
- severe rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis/polymyositis, autoimmune chronic active hepatitis, pemphigus vulgaris, polyarteritis nodosa, autoimmune haemolytic anaemia, chronic refractory idiopathic thrombocytopenic purpura
Some medicines may interfere with IBAVYR and affect how it works.
You may need to use different amounts of your medicine or you may need to take different medicines. Your doctor will advise you.
Your doctor or pharmacist has more information on medicines to be careful of or avoid using while taking IBAVYR.
Ask your doctor or pharmacist if you are not sure about this list of medicines
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect IBAVYR
4. How do I use IBAVYR?
How much to take
- The usual dose of IBAVYR depends on your weight and genotype. This can range from 600 mg to 1200 mg daily, to be taken in two divided doses (morning and evening).
- Routine blood tests will help your doctor to monitor your response to treatment.
- Your doctor may adjust your dose during therapy according to your response.
- Follow the instructions provided and use IBAVYR until your doctor tells you to stop. Take IBAVYR exactly as your doctor has directed.
- Your doctor will tell you how many tablets to take each day.
- Do not exceed the recommended dosage.
- IBAVYR has a child resistant cap. To open cap, push cap downward and twist counter clockwise.
- To close cap, place cap on bottle and turn clockwise
- IBAVYR should be taken with food.
- Do not crush or chew the tablets.
- IBAVYR 200mg tablets should be swallowed whole with water.
When to take IBAVYR
- Take IBAVYR during or immediately after a meal, at about the same times each day.
- Taking IBAVYR at the same times every day will have the best effect and help you to remember to take your tablets.
- Do not stop taking IBAVYR unless your doctor tells you to stop.
- Treatment is usually for at least 12 weeks and can go up to 24 weeks depending on your response.
- If you become pregnant while using IBAVYR Tablets, you should immediately stop taking IBAVYR and tell your doctor. If you are male and your partner becomes pregnant while you are taking IBAVYR, ask your partner to tell her doctor immediately.
If you forget to use IBAVYR
IBAVYR should be used regularly at the same time each day. If you miss your dose at the usual time, take the missed dose as soon as possible during the same day.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
- If you are not sure what to do, ask your doctor or pharmacist.
- If you have trouble remembering when to use your medicine, ask your pharmacist for some helpful hints.
If you use too much IBAVYR
If you think that you have used too much IBAVYR you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using IBAVYR?
Things you should do
Use IBAVYR Tablets exactly as your doctor has prescribed.
Tell all doctors, dentists and pharmacists who are treating you that you are taking IBAVYR.
Use contraception in order to avoid pregnancy.
Stop using IBAVYR Tablets if you become pregnant and immediately tell your doctor.
If you are male and your partner becomes pregnant while you are using IBAVYR, ask your partner to tell her doctor immediately.
Keep all your doctor's appointments so that your progress can be checked. Your doctor will carry out blood tests to monitor your response to treatment.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking IBAVYR
Things you should not do
- Do not stop taking IBAVYR or change the dose without first checking with your doctor.
- Do not use it to treat any other complaints unless your doctor says to.
- Do not give IBAVYR to anyone else, even if their symptoms seem similar to yours.
- Do not let yourself run out of medicine over weekends or holiday periods.
- Do not take any other medicines whether they require a prescription or not without first telling your doctor or pharmacist.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how IBAVYR affects you.
If you become drowsy from the combination therapy, do not drive or use machinery.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Always keep this medicine in the bottle until it is time to take it.
- Keep your medicine in a cool dry place where the temperature stays below 25°C.
- Keep bottle tightly closed.
- Heat and dampness can destroy some medicines.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking IBAVYR in combination with other oral agents.
The side effects listed below are possible side effects.
For more information please refer to the respective Consumer Medicine Information of the other oral agent used during the treatment.
Do not be alarmed by the following lists of side effects. You may not experience any of them
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. These are mild side effects of the medicine and are usually short-lived. If they continue or are severe, tell your doctor. |
Serious side effects
| Serious side effects | What to do |
signs of anaemia such as tiredness, being short of breath and looking pale
These could be signs of a serious allergic reaction.
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people. Check with your doctor as soon as possible if you have any problems while using the combination therapy, even if you do not think the problems are connected with the medicines.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What IBAVYR contains
| Active ingredient (main ingredient) | Ribavirin |
| Other ingredients (inactive ingredients) | Povidone Croscarmellose sodium Microcrystalline cellulose Crospovidone Silicon dioxide Magnesium stearate Opadry White 03F180000 (PI 109444) |
| Potential allergens | - |
Do not take this medicine if you are allergic to any of these ingredients.
What IBAVYR looks like
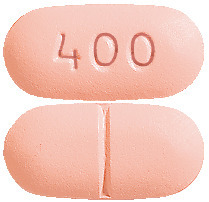
IBAVYR 200 mg tablets are white, capsule-shaped, coated tablet, debossed with "200" on one side and nothing on the other side.
The 200 mg tablets are packaged in HDPE bottles of 100 tablets.
AUST R 243632 - 200 mg tablets
Who distributes IBAVYR
In Australia and New Zealand:
Clinect Pty Ltd
120 - 132 Atlantic Drive
Keysborough VIC 3173
Australia
Free Call Australia:
1800 899 005
Free Call New Zealand:
0800 138 803
This leaflet was prepared on 14 December 2021.
® Registered trademark of PHARMASCIENCE Inc.
Published by MIMS February 2022




 When Ibavyr is used in combination treatment with any other oral agent, refer to the appropriate product information for dose reduction information.
When Ibavyr is used in combination treatment with any other oral agent, refer to the appropriate product information for dose reduction information. With the exception of anaemia and neutropenia, the majority of events presented in Table 3 occurred at severity of grade 1 in sofosbuvir containing regimens.
With the exception of anaemia and neutropenia, the majority of events presented in Table 3 occurred at severity of grade 1 in sofosbuvir containing regimens. The difference in the overall SVR rates between sofosbuvir + ribavirin and peginterferon alfa + ribavirin treatment groups was 0.3% (95% confidence interval: -7.5% to 8.0%) and the study met the predefined noninferiority criterion. Among the small number of Black/African Americans enrolled in the trial, 75% (9/12) of patients achieved SVR in the sofosbuvir + ribavirin treatment group compared to 40% (2/5) in the peginterferon alfa + ribavirin treatment group.
The difference in the overall SVR rates between sofosbuvir + ribavirin and peginterferon alfa + ribavirin treatment groups was 0.3% (95% confidence interval: -7.5% to 8.0%) and the study met the predefined noninferiority criterion. Among the small number of Black/African Americans enrolled in the trial, 75% (9/12) of patients achieved SVR in the sofosbuvir + ribavirin treatment group compared to 40% (2/5) in the peginterferon alfa + ribavirin treatment group.
 The SVR12 rate in the sofosbuvir + ribavirin treatment group was statistically significant when compared to placebo (p < 0.001).
The SVR12 rate in the sofosbuvir + ribavirin treatment group was statistically significant when compared to placebo (p < 0.001).
 Table 9 presents the subgroup analysis by genotype for cirrhosis and response to prior HCV treatment.
Table 9 presents the subgroup analysis by genotype for cirrhosis and response to prior HCV treatment.
 Table 11 presents the subgroup analysis by genotype for cirrhosis and exposure prior to HCV treatment.
Table 11 presents the subgroup analysis by genotype for cirrhosis and exposure prior to HCV treatment.
 Table 13 presents the subgroup analysis by genotype for cirrhosis.
Table 13 presents the subgroup analysis by genotype for cirrhosis.


