SUMMARY CMI
JINARC®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using JINARC?
JINARC contains the active ingredient tolvaptan. JINARC is used to treat a disease called Autosomal Dominant Polycystic Kidney Disease (ADPKD).
For more information, see Section 1. Why am I using JINARC? in the full CMI.
2. What should I know before I use JINARC?
Do not use if you have ever had an allergic reaction to JINARC or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use JINARC? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with JINARC and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use JINARC?
- JINARC is to be taken in two different doses every day.
- The dose combinations are 45 mg + 15 mg or 60 mg + 30 mg or 90 mg + 30 mg.
More instructions can be found in Section 4. How do I use JINARC? in the full CMI.
5. What should I know while using JINARC?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using JINARC? in the full CMI.
6. Are there any side effects?
Common side effects include thirst; increased amount and frequency of urination; headache; constipation, diarrhoea, dry mouth, indigestion, decreased appetite; fatigue, weakness, dizziness; trouble sleeping; muscle spasms; rash, dry skin, itching; painful, swollen joints. Serious side effects include difficulty urinating; swelling of the face, lips or tongue, generalised rash, or severe wheezing or breathlessness; signs of electrolyte imbalances.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
JINARC®
Active ingredient(s): tolvaptan (tol-vap-tan)
JINARC can cause serious liver problems.
Stop taking JINARC and call your healthcare provider right away if you get any of the following symptoms: feeling tired, fever, loss of appetite, rash, nausea, itching, right upper stomach (abdomen) pain or tenderness, yellowing of the skin and white part of the eye (jaundice), vomiting, dark urine.
To help reduce your risk of liver problems, your healthcare provider will ask you to do a blood test to check your liver before you start taking JINARC, monthly for the first 18 months of treatment and then every 3 months.
It is important to stay under the care of your healthcare provider during treatment with JINARC.
Consumer Medicine Information (CMI)
This leaflet provides important information about using JINARC. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using JINARC.
Where to find information in this leaflet:
1. Why am I using JINARC?
2. What should I know before I use JINARC?
3. What if I am taking other medicines?
4. How do I use JINARC?
5. What should I know while using JINARC?
6. Are there any side effects?
7. Product details
1. Why am I using JINARC?
JINARC contains the active ingredient tolvaptan.
JINARC belongs to a group of medicines called vasopressin antagonists.
This means that it prevents a hormone called vasopressin from binding to receptors in your kidneys. By blocking the effect of vasopressin, JINARC slows the development of kidney cysts in patients with ADPKD, reduces symptoms of the disease and increases urine production.
JINARC is used to treat a disease called Autosomal Dominant Polycystic Kidney Disease (ADPKD). This disease causes growth of cysts in the kidneys which results in problems because of their size and the space they occupy.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
This medicine is available only with a doctor's prescription.
Jinarc is not recommended for use in children and teenagers, or patients older than 55 years. The effects of Jinarc in people younger than 18 years have not been studied, and in patients older than 55 years have not been proven.
2. What should I know before I use JINARC?
Warnings
Do not use JINARC if:
- you are allergic to tolvaptan, benzazepine derivatives or any of the ingredients listed at the end of this leaflet
- you have been told that you have raised levels of liver enzymes in your blood
- you have high level of sodium in your blood ("hypernatraemia")
- you do not realise when you are thirsty
- your kidneys cannot produce urine
- you have low blood volume
- you are pregnant
- you are breastfeeding
- you are below the age of 18 years
Safety and effectiveness in children younger than 18 years have not been established.
Always check the ingredients to make sure you can take this medicine
Check with your doctor if you:
- have any other medical conditions
- take any medicines for any other condition
- cannot drink enough water or if you are fluid restricted
- have difficulties in urination or have an enlarged prostate
- suffer from liver disease
- suffer from too high or too low blood sodium
- suffer from high potassium levels in your blood
- have diabetes
- have high blood pressure and are taking medications to treat it
- are dehydrated or suffer from excessive vomiting, diarrhoea or sweating
- have gout
- have lactose intolerance
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or plan to become pregnant.
Tell your doctor if you are breastfeeding or plan to breastfeed.
JINARC must not be taken if you are pregnant or while breastfeeding.
You must use a reliable method of contraception to avoid becoming pregnant while you are taking JINARC. You should continue doing this for one month after stopping treatment.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop. In particular let your doctor know if you are taking:
- treatments containing ketoconazole, fluconazole or itraconazole for fungal infections
- antibiotics such as clarithromycin, erythromycin, and ciprofloxacin
- medicines for the treatment of HIV such as saquinavir, ritonavir and atazanavir
- medicines for high blood pressure and chest pain such as diltiazem and verapamil
- medicines which increase the level of sodium in your blood or which contain large amounts of salt, like tablets that dissolve in water and indigestion remedies
- digoxin, a medicine for the treatment of irregular heart beat and heart failure
- cyclosporine, a medicine that reduces the immune response
- quinidine, a medicine used for malaria
- dabigatran, a medicine used to thin the blood
- rosuvastatin and pitavastatin, medicines used to lower cholesterol
- methotrexate, a medicine used to treat rheumatoid arthritis
- sulfasalazine, a medicine used to treat inflammatory bowel disease
- metformin, a medicine for diabetes
- medicines for the treatment of epilepsy such as phenytoin and carbamazepine
- rifampicin, an antibiotic
- St John's wort, a traditional herbal medicinal product for the relief of slightly low mood and mild anxiety
- fluid tablets and other medicines used for the treatment of high blood pressure
- desmopressin, a medicine used to control urine output or bedwetting
It may still be alright for you to take these medicines and JINARC together. Your doctor will be able to decide what is suitable for you.
JINARC with food and drink
Do not drink grapefruit juice when taking JINARC.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect JINARC.
4. How do I use JINARC?
How much to take / use
- JINARC is to be taken in two different doses every day
- The dose combinations are 45 mg + 15 mg or 60 mg + 30 mg or 90 mg + 30 mg
- One tablet of the higher dose (45 mg, 60 mg or 90 mg) should be taken in the morning upon waking, at least 30 minutes before food and one tablet with the lower dose (15 mg or 30 mg) should be taken 8 hours later. The afternoon dose can be taken with or without food.
- Your doctor will start with a dose combination of 45 mg in the morning and 15 mg eight hours later and may then increase it to a maximum of 90 mg in the morning and 30 mg after 8 hours
- To find the best dosage for you, your doctor will regularly examine whether you tolerate a prescribed dose. You should always take the highest tolerable dose combination prescribed by your doctor.
- If you take other medicines which can increase the effects of JINARC, you may receive lower JINARC doses
- Your doctor may have prescribed a different dose
- Follow the instructions provided and use JINARC until your doctor tells you to stop
How to take JINARC
- Swallow the tablets without chewing, with a glass of water
- The morning dose should be taken at least 30 minutes before the morning meal. The second daily dose can be taken with or without food.
- Do not chew, crush or split the tablets. To ensure you get the entire dose, the tablets should be swallowed whole without chewing or crushing.
When to take / use JINARC
- JINARC should be taken at about the same time each day
- Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
- Your first dose should be taken in the morning and your second dose approximately 8 hours later
If you forget to use JINARC
JINARC should be used regularly at the same time each day. If you miss your dose at the usual time, you should take the dose as soon as you remember on the same day.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you use too much JINARC
If you think that you have used too much JINARC, you may need urgent medical attention.
In Australia, you should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital
In New Zealand, you should immediately:
- phone the National Poisons Centre (by calling 0800 POISON or 0800 764 766), or
- contact your doctor, or
- go to accident and emergency at your nearest hospital
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using JINARC?
Things you should do
MAKE SURE YOU DRINK ENOUGH WATER
- JINARC causes water loss because it increases your urine production. You may experience urine loss of between 5-7 L per day.
- This water loss may result in side effects such as dry mouth and thirst or even more severe side effects like kidney problems (see "Side Effects"). It is therefore important that you have access to water and that you are able to drink sufficient amounts when you feel thirsty.
- Unless your doctor tells you otherwise, drink plenty of water during the day and 1 or 2 glasses before going to bed, even if you do not feel thirsty and you must also drink water after you urinate at night.
- Exposure to prolonged heat and humidity, exercise and intercurrent illness can further increase your risk of dehydration. In these circumstances, you should drink more water or fluid to reduce your risk of dehydration.
- Special care must be taken if you have a disease that reduces appropriate fluid intake or if you are at an increased risk of water loss e.g. in case of vomiting or diarrhoea. If this occurs stop your tolvaptan treatment and seek medical advice immediately.
- Due to the increased urine production it is also important that you always have access to a toilet.
Call your doctor straight away if you:
- become pregnant while taking JINARC. Your doctor will advise you on whether you should stop treatment. Your doctor will advise you on whether you should stop treatment.
Remind any doctor, dentist or pharmacist you visit that you are using JINARC.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking JINARC. It may affect other medicines used during surgery.
If you are about to have any blood tests, tell your doctor that you are taking JINARC.
It may interfere with the results of some tests.
During treatment with JINARC, your doctor will arrange regular (e.g. monthly) blood tests to check for changes in your liver function.
Keep all of your doctor's appointments so that your progress can be checked.
Things you should not do
- Do not stop taking this medicine or lower the dosage without checking with your doctor.
- Do not take JINARC to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
Your doctor will carefully observe you when you start JINARC, especially when your dose if being increased.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how JINARC affects you.
JINARC may cause side effects that can affect your ability to drive or use machines.
JINARC may make you feel dizzy or sleepy, particularly at the beginning of treatment. If this happens to you, do not drive or use any tools or machines.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep your medicine in the original container.
- If you take it out of its original container it may not keep well.
- Keep your medicine in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
General well-being related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Allergy related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
JINARC may cause your liver to not work properly. Therefore, please inform your doctor immediately if you have any signs that could indicate potential liver problems, such as:
- nausea
- vomiting
- fever
- tiredness
- loss of appetite
- pain in the abdomen
- dark urine
- jaundice (yellowing of the skin or eyes)
- itching of your skin
- joint and muscle pain with fever
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
In Australia, after you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What JINARC contains
| Active ingredient (main ingredient) |
|
| Other ingredients (inactive ingredients) |
|
| Potential allergens | This medicine does not contain gluten, tartrazine or any other azo dyes |
Do not take this medicine if you are allergic to any of these ingredients.
What JINARC looks like
The different strengths of JINARC tablets have different shapes and embossing:
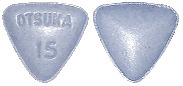
- 15 mg tablet: blue, triangular, debossed with “OTSUKA” and “15” on one side
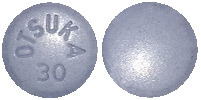
- 30 mg tablet: blue, round, debossed with “OTSUKA” and “30” on one side (Aust R 272786)
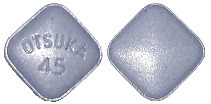
- 45 mg tablet: blue, square, debossed with “OTSUKA” and “45” on one side (Aust R 272785)
- 60 mg tablet: blue, modified rectangular, debossed with “OTSUKA” and “60” on one side
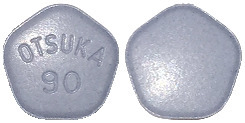
- 90 mg tablet: blue, pentagonal, debossed with “OTSUKA” and “90” on one side
Australian Registration Details:
JINARC 15 mg tablet blister pack - Aust R 272785
JINARC 30 mg tablet blister pack - Aust R 272786
JINARC 15 mg + 45 mg tablet blister composite pack
- Aust R 272787
JINARC 30 mg + 60 mg tablet blister composite pack
- Aust R 272788
JINARC 30 mg + 90 mg tablet blister composite pack
- Aust R 272789
Who distributes JINARC
In Australia:
Otsuka Australia Pharmaceutical Pty Ltd
Suite 2.03, Level 2
9 Help Street, Chatswood NSW 2067
Under the licence of Otsuka Pharmaceutical Co., Ltd.
In New Zealand:
Pharmacy Retailing (NZ) Ltd trading as Healthcare Logistics
58 Richard Pearse Drive
Airport Oaks, Mangere
Auckland 2022
Phone number: 0800 602 200
This leaflet was prepared in October 2022.
Published by MIMS December 2022



 Further reductions have to be considered if patients cannot tolerate the reduced tolvaptan doses.
Further reductions have to be considered if patients cannot tolerate the reduced tolvaptan doses.

 The next sequentially ordered secondary endpoint of slope of kidney function decline was assessed as change in estimated glomerular filtration rate (eGFR-CKD EPI) during treatment (from end of titration to last on-drug visit). The tolvaptan-treated patients had a 26.4% reduction in the rate of renal function decline compared with placebo (-2.7 versus -3.7 (mL/min/1.73 m2), p < 0.0001, Figure 2). Figure 2 represents slope of renal function for tolvaptan (solid) and placebo (dashed) change from end of titration baseline (i.e. end of Week 3). Box plots derived from mixed effect model repeat measurement (MMRM) analyses to each indicated 12-month visit with 5th, 25th, mean, 75th and 95th percentiles of change from end of titration for tolvaptan (grey) and placebo (white) groups.
The next sequentially ordered secondary endpoint of slope of kidney function decline was assessed as change in estimated glomerular filtration rate (eGFR-CKD EPI) during treatment (from end of titration to last on-drug visit). The tolvaptan-treated patients had a 26.4% reduction in the rate of renal function decline compared with placebo (-2.7 versus -3.7 (mL/min/1.73 m2), p < 0.0001, Figure 2). Figure 2 represents slope of renal function for tolvaptan (solid) and placebo (dashed) change from end of titration baseline (i.e. end of Week 3). Box plots derived from mixed effect model repeat measurement (MMRM) analyses to each indicated 12-month visit with 5th, 25th, mean, 75th and 95th percentiles of change from end of titration for tolvaptan (grey) and placebo (white) groups. Subgroup analysis of all endpoints above (change in TKV, key composite [including time to worsening of kidney function and time to medically significant kidney pain] and change in slope of decline in renal function) demonstrated consistent efficacy (directional) in all pre-specified subgroups, including those stratified by age, gender, race, geographical location, baseline hypertension, baseline eGFR and baseline TKV.
Subgroup analysis of all endpoints above (change in TKV, key composite [including time to worsening of kidney function and time to medically significant kidney pain] and change in slope of decline in renal function) demonstrated consistent efficacy (directional) in all pre-specified subgroups, including those stratified by age, gender, race, geographical location, baseline hypertension, baseline eGFR and baseline TKV. The key secondary endpoint was a comparison of the efficacy of tolvaptan treatment versus placebo in reducing the decline of annualised eGFR slope across all measured time points in the trial. These data also showed statistically significant benefit from tolvaptan vs. placebo (p < 0.0001) (Table 6).
The key secondary endpoint was a comparison of the efficacy of tolvaptan treatment versus placebo in reducing the decline of annualised eGFR slope across all measured time points in the trial. These data also showed statistically significant benefit from tolvaptan vs. placebo (p < 0.0001) (Table 6). Prespecified subgroup analyses of the primary endpoint showed a beneficial effect of tolvaptan across subgroups that were defined according to sex, baseline estimated GFR, stage of chronic kidney disease (except for stage 2) and geographic region, as well as in the subgroups of patients who were 55 years of age or younger and patients who were white.
Prespecified subgroup analyses of the primary endpoint showed a beneficial effect of tolvaptan across subgroups that were defined according to sex, baseline estimated GFR, stage of chronic kidney disease (except for stage 2) and geographic region, as well as in the subgroups of patients who were 55 years of age or younger and patients who were white. Data are not currently available to show whether long-term therapy with Jinarc continues to slow the rate of renal function decline and affect clinical outcomes of ADPKD, including delay in the onset of end-stage renal disease.
Data are not currently available to show whether long-term therapy with Jinarc continues to slow the rate of renal function decline and affect clinical outcomes of ADPKD, including delay in the onset of end-stage renal disease. Chemical Name: (±)-4'-[(7-chloro- 2,3,4,5-tetrahydro -5-hydroxy-1H-1-benzazepin -1-yl) carbonyl]-o-tolu-m-toluidide.
Chemical Name: (±)-4'-[(7-chloro- 2,3,4,5-tetrahydro -5-hydroxy-1H-1-benzazepin -1-yl) carbonyl]-o-tolu-m-toluidide.