What is in this leaflet
This leaflet answers some common questions about Magicul.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking Magicul against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What Magicul is used for
Magicul is used to:
- treat stomach and duodenal ulcers (duodenal ulcers occur in the tube leading out of the stomach)
- prevent stomach and duodenal ulcers from coming back
- treat gastro-oesophageal reflux disease which is the washing back of food and acid from the stomach up into the food pipe (oesophagus)
- relieve heartburn and other symptoms of reflux disease
- treat Zollinger-Ellison syndrome, a rare disorder when the stomach produces very large amounts of acid, much more than in ulcers and reflux disease
- treat scleroderma oesophagitis, a rare condition in which the food pipe becomes thick and hardened, allowing acid to reflux.
How Magicul works
Magicul belongs to a group of medicines called H2-antagonists or H2-blockers. These medicines work by reducing the amount of acid made by the stomach. This helps to:
- relieve heartburn, the chest pain or burning sensation rising up the food pipe
- heal the ulcer and/or reflux disease.
Your doctor may have prescribed Magicul for another reason. Ask your doctor if you have any questions about why Magicul has been prescribed for you.
Magicul is available only with a doctor's prescription.
There is no evidence that Magicul is addictive.
Before you take Magicul
When you must not take it
Do not take Magicul if you are allergic to medicines containing cimetidine or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include skin rash, itching or hives, swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing, wheezing or shortness of breath.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
Magicul is not recommended for use in children unless advised by your child's doctor, as there have been limited studies of its effects in children of this age group.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking Magicul during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. Magicul passes into breast milk. Your doctor will discuss the risks and benefits of taking Magicul when breastfeeding.
Tell your doctor if you have, any medical conditions, especially the following:
- liver problems
- kidney problems
- lung disease
- diabetes
- lowered immunity.
Your doctor may want to take special care if you have any of these conditions.
If you have not told your doctor about any of the above, tell them before you start taking Magicul.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and Magicul interfere with each other. These include:
- warfarin, a medicine used to prevent blood clots
- phenytoin, a medicine used to treat epilepsy
- theophylline, a medicine used to treat asthma
- metformin, a medicine used to treat diabetes
- chlormethiazole, a sedating medicine used in alcohol withdrawal and some other conditions
- medicines used for high blood pressure and some heart conditions
- medicines used to treat depression or anxiety
- sleeping tablets
- medicines used to relieve arthritis or other pain
- ciclosporin and tacrolimus, medicines used to prevent rejection of transplanted organs
- atazanavir, a medicine used to treat HIV infection
- some medicines used to treat fungal infections, such as ketoconazole, itraconazole or posaconazole
- narcotic analgesics, such as morphine or codeine
- medicines used to treat cancer.
These medicines may be affected by Magicul or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Some antacids may interfere with the absorption of Magicul.
To make sure there is no problem with absorption, you should take Magicul at least one hour before or one hour after taking antacids.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Magicul
How much to take
To treat an ulcer or reflux disease, the usual dose is 800 mg a day. This can be taken as 800 mg at bedtime or as 400 mg in the morning and 400 mg at bedtime.
To prevent an ulcer from coming back, the usual dose is 400 mg at bedtime.
For short-term relief of heartburn or other symptoms of reflux disease, the usual dose is 200 mg up to four times a day. The maximum dose is 800 mg a day. Magicul does not provide immediate relief of symptoms.
Your doctor may advise you to take a different dose. This depends on your condition and whether you are taking any other medicines.
Follow all directions given to you by your doctor and pharmacist carefully.
How to take it
Swallow the tablets whole with a glass of water.
Do not crush or chew the tablets. If you crush or chew Magicul tablets, they will not work as well.
When to take it
Take Magicul at about the same time each day. Taking your medicine at the same time each day will give the best effect and help you to remember when to take your medicine.
If your doctor has prescribed divided doses of Magicul, space them evenly apart throughout the day. Magicul can be taken with or without food.
If you are undergoing haemodialysis, Magicul should be taken after dialysis.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
How long to take it for
If you are taking Magicul to heal an ulcer, you will need to take it for 4 to 8 weeks.
If you are taking Magicul to treat reflux disease, you may need to take it for up to 12 weeks.
For short-term relief of heartburn and symptoms of reflux disease, you can take Magicul for up to 2 weeks. If the pain or symptoms are not relieved by Magicul or persist you should see your doctor.
If you are taking Magicul to stop an ulcer from coming back or to treat other conditions, your doctor will tell you how long you need to take the tablets.
Keep taking Magicul for as long as your doctor recommends.
It is very important that you take the full course of Magicul prescribed by your doctor so that your condition is properly treated.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much Magicul. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Magicul, you may feel drowsy or confused.
While you are taking Magicul
Things you must do
If you are about to be started on any new medicine, tell your doctor or pharmacist that you are taking Magicul.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all your doctor's appointments so that your progress can be checked. Your doctor may want you to have tests to make sure that your ulcer or reflux disease is healing.
Things you must not do
Do not stop taking Magicul, or change the dose, without checking with your doctor.
Do not use Magicul to treat any other complaints unless your doctor tells you to.
Do not give Magicul to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how Magicul affects you. Magicul may cause drowsiness or dizziness in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Suggestions that may help your condition
Some self-help actions suggested below may help your condition. Talk to your doctor or pharmacist about these and ask for more information.
- Alcohol - your doctor may advise you to limit your alcohol intake.
- Aspirin and many other medicines used to treat arthritis, period pain and headaches - these medicines may irritate the stomach and may make your condition worse. Your doctor or pharmacist may suggest other medicines you can take.
- Caffeine - your doctor may advise you to limit the number of drinks which contain caffeine, such as coffee, tea, cocoa and cola drinks, because they contain ingredients that may irritate your stomach.
- Eating habits - eat smaller, more frequent meals. Eat slowly and chew your food carefully. Try not to rush at meal times.
- Smoking - your doctor may advise you to stop smoking or at least cut down.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Magicul. Like all other medicines, Magicul may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by the following list of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- tiredness, drowsiness
- dizziness
- muscle aches and pains
- mild skin rash
- hair loss
- diarrhoea, constipation, wind
- nausea (feeling sick), vomiting
- in men, enlarged breasts and impotence (these have been reported rarely in patients receiving high doses).
For the most part, these side effects are mild.
Tell your doctor immediately if you notice any of the following:
- fast, slow or irregular heart beat
- yellowing of the eyes or skin (jaundice)
- fever
- confusion, hallucinations, depression
- severe upper stomach pain, often with nausea and vomiting
- unusual bruising or bleeding
- looking pale, tiredness, being short of breath when exercising
- signs of frequent infections such as fever, chills, sore throat or mouth ulcers.
These side effects may be serious. You may need urgent medical attention.
If any of the following happen, stop taking Magicul and tell your doctor immediately, or go to Accident and Emergency at the nearest hospital:
- hives or severe skin reactions
- swelling of the face, mouth, throat or limbs
- shortness of breath or difficulty breathing.
These reactions may be signs of a severe allergic reaction to Magicul. Allergic reactions are rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell. Other side effects not listed above may also occur in some people.
Some health problems may arise from the condition being treated itself, rather than the treatment. For this reason, contact your doctor immediately if you notice any of the following:
- pain or indigestion which occurs during treatment with Magicul
- vomiting
- passing black or blood-stained motions
- unexpected weight loss.
After taking Magicul
Storage
Keep Magicul where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in a cool dry place where the temperature stays below 30°C for 200 mg and 800 mg tablets, and below 25°C for 400 mg tablets
Do not store Magicul or any other medicine in the bathroom or near a sink.
Do not leave Magicul in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking Magicul, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
Magicul comes in 3 strengths of tablets:
- Magicul 200 - round, pale green, film-coated tablet marked CE2 on one side and a Greek Alpha symbol on the reverse. Each pack contains 120 tablets.
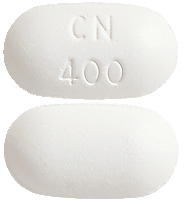
- Magicul 400 mg - White film coated biconvex tablets embossed "CN 400" on one side with "G" on the reverse. Each pack contains 60 tablets.
- Magicul 800 - oval, pale green, film-coated tablet marked CE8 on one side and a Greek Alpha symbol on the reverse. Each pack contains 30 tablets.
Ingredients
The active ingredient in Magicul is cimetidine. Each tablet contains 200 mg, 400 mg or 800 mg of cimetidine.
The tablets also contain:
- maize starch
- povidone
- microcrystalline cellulose
- sodium starch glycollate (Type A)
- magnesium stearate
- Opadry Green OY-8830 (ARTG no. 1538) for 200 mg and 800 mg tablets.
- Opadry White Y-1-7000 E171 (ARTG no. 2731) for 400 mg tablets.
The tablets are gluten free.
Manufacturer
Magicul is supplied in Australia by:
Alphapharm Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration number:
Magicul 400 - AUST R 213382
This leaflet was prepared in December 2019.
Magicul_cmi\Dec19/00
Published by MIMS March 2020


 For patients undergoing haemodialysis it is recommended that dialysis be carried out just prior to the next scheduled dosage since some drug will be removed by dialysis. Where circumstances require an increase in dosage, increases should be made by increasing the frequency of administration of 200 mg doses.
For patients undergoing haemodialysis it is recommended that dialysis be carried out just prior to the next scheduled dosage since some drug will be removed by dialysis. Where circumstances require an increase in dosage, increases should be made by increasing the frequency of administration of 200 mg doses.