What is in this leaflet
This leaflet answers some common questions about Maxolon.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Maxolon against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Maxolon is used for
In adults over 20 years this medicine is used to:
- treat nausea and vomiting caused by infectious diseases, migraine, kidney disease, child birth, other medications, cancer, or following surgery, chemotherapy or radiation treatment.
- activate stomach contractions in conditions where there is a need to encourage normal passage of food through the stomach and intestines.
- with X-rays to help diagnose problems of the stomach and/or intestines.
- help with passing tubes into the intestine.
In young adults and children over 1 year of age this medicine is used to:
- treat severe vomiting of known cause or following chemotherapy or radiation treatment.
- help with passing tubes into the intestine.
This medicine works by blocking the action of a chemical in the brain which causes nausea and vomiting. It also acts in the stomach and upper intestine to increase muscle contractions.
Ask your doctor if you have any questions about why this medicine has been prescribed for you or your child. Your doctor may have prescribed it for another reason.
Before taking Maxolon
When you must not take it
Do not take Maxolon if you have an allergy to:
- any medicine containing Metoclopramide
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Maxolon if you have any of the following:
- active bleeding from the stomach and/or digestive tract.
- blockage of the stomach and/or digestive tract.
- recent surgery of the stomach and/or digestive tract.
- phaeochromocytoma (an adrenaline producing tumour of the adrenal gland).
- epilepsy (fits or seizures).
- take other medication such as antipsychotic/neuroleptic medication and certain antidepressants that can cause movement disorders (extrapyramidal reactions).
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before starting to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- Parkinson's disease
- liver or kidney problems
- high blood pressure
- asthma
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start taking Maxolon.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Maxolon may interfere with each other. These include:
- tranquilizers or anti-anxiety medications
- strong pain relievers (e.g. codeine or morphine)
- sedatives or sleeping medication
- atropine-like medications (e.g. some cold preparations, travel sickness medicines)
- tetracycline antibiotics, paracetamol, levodopa
- digoxin
These medicines may be affected by Maxolon or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take Maxolon
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Follow your doctor's instructions about how much Maxolon to use. The dose of Maxolon varies with the age of the patient and the reason for use.
The total daily dosage of Maxolon, especially for children and young adults, should not normally exceed 0.5mg/kg bodyweight or 30mg daily.
Space the doses as evenly as possible throughout the day.
Tablets
- 20 + years - 1 tablet every 8 hours
- 15 to 20 years - ½ to 1 tablet every 8 hours
- 1 to 14 years - Use Ampoules Only
Ampoules Your doctor will decide the dose of Maxolon ampoules to be given and how long it is to be administered.
The usual dose of Maxolon is:
- 20 + years - 10 mg every 8 hours
- 15 to 20 years - 5 to 10 mg every 8 hours
- 5 to 14 years - 2.5 to 5 mg every 8 hours
- 3 to 5 years - 2 mg every 8 to 12 hours
- 1 to 3 years - 1 mg every 8 to 12 hours
Children and young adults are very sensitive to the effects of Maxolon. Your doctor will normally start treatment at the lower dose. Do not exceed the prescribed dose in these age groups.
How to take it
Tablets:
Swallow the tablets with a full glass of water.
The tablets can be broken in half (along the break-line).
Do not exceed the prescribed dose.
Ampoules:
Your doctor or nurse will inject the necessary dose of Maxolon.
It may be given by injection in the muscle of the upper arm, buttock or into a vein. The doctor will decide the best method of injection.
When to take it
Take Maxolon at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take Maxolon 30 minutes before meals.
How long to take it
Continue taking Maxolon for as long as your doctor tells you.
If you forget to take it
Take it as soon as you remember, and then go back to taking the medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia 13 11 26, New Zealand 0800 764 766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Maxolon. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include drowsiness, confusion, dizziness, headache, agitation, nausea, vomiting, constipation, tremor, twitching or uncontrolled spasm of muscles.
While using Maxolon
Things you must do
If vomiting or nausea persist, tell your doctor.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Maxolon.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
Things you must not do
Do not use Maxolon to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition.
Things to be careful of
Be careful driving or operating machinery until you know how Maxolon affects you. This medicine may cause dizziness, light-headedness, tiredness or drowsiness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous. Children should be careful when riding bicycles or climbing trees.
Be careful when drinking alcohol while you are taking this medicine. If you drink alcohol it may make you sleepy.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Maxolon.
This medicine may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- drowsiness, tiredness
- restlessness
- dizziness, headache
- bowel irregularities
- insomnia
- anxiety
- agitation
The above list includes the more common side effects of your medicine. They are usually mild and short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- uncontrolled or repeated movements, e.g. sucking or smacking of the lips, darting of the tongue, chewing movements, uncontrolled movements of the arms or legs. This may be a sign of tardive dyskinesia, a movement disorder which can be potentially irreversible.
- fast heartbeat
- depression
- swelling of hands, ankles or feet
The above list includes serious side effects that may require medical attention. Serious side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- allergic reaction including fainting, swelling of limbs, face, lips, mouth or throat which may cause difficulty swallowing or breathing.
- sudden uncontrolled muscle spasm, stiffness of the arms or legs, muscle spasm of the face, locked-jaw or upturned eyes.
- shuffling walk, slowing of all movement, muscle tremor.
- neuroleptic malignant syndrome, a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell. Other side effects not listed above may also occur in some people.
After taking Maxolon
Storage
Keep your medicine in the pack until it is time to take it. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Maxolon ampoules will be stored in the pharmacy or on the ward. The injection is kept in a cool dry place where the temperature stays below 25°C.
Do not store Maxolon or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Maxolon ampoules are usually given in a hospital setting. Your pharmacist will dispose of any left over Maxolon.
Product description
What it looks like
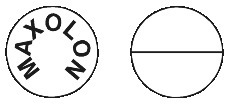
Maxolon tablets: round white tablets marked with Maxolon on one side and a break line on the other side.
Ampoules: clear colourless solution
Ingredients
Maxolon tablets contain 10 mg of metoclopramide as the active ingredient.
It also contains:
- starch - maize
- silica - colloidal anhydrous
- magnesium stearate
- starch - pregelatinised maize
- lactose
Maxolon injection supplied in ampoules contain 10mg/2mL of metoclopramide as the active ingredient.
It also contains:
- Sodium chloride
- Water for injections - purified
Maxolon preparations do not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Maxolon is supplied in Australia by:
iNova Pharmaceuticals (Australia) Pty Ltd
ABN: 88 000 222 408
Level 10, 12 Help Street
Chatswood NSW 2067
Tel: 1800 253 272
Maxolon tablets are supplied in New Zealand by:
Bausch & Lomb (NZ) Ltd,
c/- Radiant Health Limited
Pier 21, Level 3, 11 Westhaven Drive, Auckland Central 1010.
Toll free number: 0508375394
® Registered Trademark
Maxolon 10 mg tablets
AUST R 11153
Maxolon 10mg/2mL injection
AUST R 40204
This leaflet was updated in March 2015.
Published by MIMS August 2015