What is in this leaflet
This leaflet answers some common questions about METEX XR.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking METEX XR against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor, pharmacist or diabetes educator.
Keep this leaflet with your medicine. You may need to read it again.
What METEX XR is used for
METEX XR is used to control blood glucose (sugar) in patients with Type 2 diabetes mellitus, especially in those who are overweight. It is used when diet and exercise are not enough to control high levels of blood glucose.
METEX XR can be used alone, or in combination with other medicines for treating diabetes.
TYPE 2 DIABETES MELLITUS
Type 2 diabetes mellitus is also called non-insulin dependent diabetes mellitus (NIDDM) or maturity onset diabetes.
Insulin is a hormone that enables body tissues to take up glucose from the blood and use it for energy or fat storage for future use.
People with Type 2 diabetes are unable to make enough insulin or their body does not respond properly to the insulin it does make. This causes a build-up of glucose in the blood (hyperglycaemia), which can lead to serious medical problems.
Long term hyperglycaemia can lead to heart disease, blindness, kidney damage, poor blood circulation and gangrene.
Signs of hyperglycaemia include:
- tiredness or lack of energy
- headache
- thirst
- passing large amounts of urine
- blurred vision
How METEX XR works
METEX XR belongs to a group of medicines called biguanides. It lowers high blood glucose levels by:
- improving your body’s sensitivity to insulin and restoring the way it normally uses glucose
- reducing the amount of glucose your liver makes
- delaying the amount of glucose your intestine absorbs.
Ask your doctor if you have any questions about why METEX XR has been prescribed for you.
METEX XR is not recommended in children as its safety and effectiveness have not been established in this age group.
METEX XR is available only with a doctor’s prescription.
There is no evidence that METEX XR is addictive.
This medicine has been prescribed for you personally and you should not pass it onto others even if their symptoms are the same as yours.
Before you take METEX XR
When you must not take it
Do not take METEX XR if you are allergic to:
- medicines containing metformin (such as Diaformin, Glucophage) or any other biguanide
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing; wheezing or shortness of breath.
Do not take METEX XR if you have any of the following medical conditions:
- Type 1 diabetes mellitus that is well controlled by insulin alone
- Type 2 diabetes that is already controlled by diet alone
- serious complications with your diabetes or any type of metabolic acidosis, diabetic ketoacidosis (a symptom of uncontrolled diabetes, in which substances called ketone bodies accumulate in the blood - you may notice this as an unusual fruity odour on your breath)
- kidney failure or severe kidney disease
- dehydration (for instance due to persistent or severe vomiting or diarrhoea)
- shock from severe injury or blood loss
- severe liver disease
- acute alcohol intoxication, chronic alcohol dependence
- certain heart or blood circulation problems, including a recent heart attack or heart failure (when heart fails to pump blood effectively)
- blood clots in the lungs (symptoms include coughing shortness of breath, chest pain, and a fast heart rate), severe breathing difficulties
- inflammation of the pancreas (symptoms include severe upper stomach pain, often with nausea and vomiting) if associated with severe infection or hypoxia (lack of oxygen).
- a severe infection or gangrene.
Do not take METEX XR if you need to have major surgery or an examination such as an X-ray or a scan requiring an injection of iodinated contrast (dye). You must stop taking METEX XR for a certain period of time before and after the examination or the surgery. Your doctor will decide whether you need any other treatment for this time. It is important that you follow your doctor's instructions precisely.
Do not take this medicine if you are pregnant or plan to become pregnant. Insulin is more suitable for controlling blood glucose during pregnancy. Your doctor will replace METEX XR with insulin while you are pregnant.
Do not take it if you are breastfeeding. Your doctor will discuss the options available to you.
Do not take METEX XR after the expiry date (Exp.) printed on the pack, or if the packaging is torn or shows signs of tampering. If it has expired or if the packaging is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking METEX XR, ask your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, dyes or preservatives.
Before starting METEX XR your doctor will ask you to have a blood test to check your kidney function.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney problems
- liver problems
- heart or blood vessel problems including heart failure.
Tell your doctor if you drink alcohol. Alcohol can affect the control of your diabetes. Drinking excessive amounts of alcohol while you are being treated with METEX XR may also lead to serious side effects.
Your doctor may suggest you stop drinking or reduce the amount of alcohol you drink.
If you have not told your doctor about any of the above, tell him/her before you start taking METEX XR.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and METEX XR may interfere with each other.
These include:
- other medicines used to treat diabetes such as insulin, glitinides (Novonorm), and sulfonylureas (e.g. Amaryl, Daonil, Diamicron, Glimel, Glyade, Melizide, Minidiab)
- iodinated contrast agents (dyes)
- medicines that contain alcohol, such as cough and cold syrups
- corticosteroids such as prednisone (Panafacort, Sone) and cortisone (Cortate)
- tetracosactrin, a medicine used in people with multiple sclerosis, and in young children to treat some types of seizures (fits)
- danazol, a medicine used to treat Endometriosis
- medicines used to treat high blood pressure and some heart conditions, such as beta-blockers, metoprolol (e.g. Betaloc, Minax), calcium channel blockers such as nifedipine (e.g. Adalat, Adefin), ACE inhibitors such as captopril (e.g. Capoten, Acenorm), enalapril (e.g. Alphapril, Amprace, Renitec) fosinopril (Monopril), lisinopril (e.g. Lisodur, Prinivil, Zestril), perindopril (Coversyl), quinapril (Accupril, Asig).
- some medicines used to treat asthma such as salbutamol (Ventolin) or terbutaline (Bricanyl).
- diuretics, also called fluid tablets, such as amiloride (Midamor, Kaluril), bumetanide (Burinex), frusemide (Lasix, Uremide, Urex), hydrochlorothiazide (Dithiazide), spironolactone (Aldactone, Spiractin).
- Chlorpromazine, a medicine used to treat schizophrenia and other mental illnesses
- NSAIDs (non-steroidal anti-inflammatory drugs), medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis such as aspirin (e.g. Disprin, Solprin), diclofenac (e.g. Voltaren, Fenac), ibuprofen (e.g. Actiprofen, Brufen, Rafen), meloxicam (Mobic), naproxen (e.g. Naprogesic, Naprosyn, Inza) and piroxicam (e.g. Feldene, Mobilis)
- medicines used to treat ulcers and reflux, such as cimetidine (e.g. Tagamet, Magicul)
- medicines used to prevent blood clots such as warfarin (e.g. Coumadin, Marevan)
- thyroid hormones such as thyroxine (e.g. Oroxine, Eutoxsig)
- medicines that are substrates/inhibitors of organic cation transporters - OCT 1 such as dolutegravir, crizotinib, olaparib, daclatasvir or vandetanib
- medicines that are inducers of OCT 1 such as rifampicin
- medicines that may increase the risk of lactic acidosis when concomitantly used with metformin hydrochloride such as topiramate and other carbonic anhydrase inhibitors.
These medicines may be affected by METEX XR or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking METEX XR.
How to take METEX XR
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist.
How much to take
METEX XR comes is available on two strengths:
METEX XR 500 (500 mg Tablets)
and
METEX XR 1000 (1000 mg Tablets).
The dose varies from person to person. Your doctor will decide the right dose for you. The usual starting dose is 1 tablet (500 mg) once daily with the evening meal. Your doctor may increase the dose slowly, depending on your blood glucose levels.
The maximum recommended dose is 2 grams once per day.
The elderly and people with kidney problems may need smaller doses.
How to take METEX XR
Swallow the tablets with a glass of water.
Do not break, crush or chew the tablets. If you break, crush or chew METEX XR, they will not work as well. METEX XR are modified release tablets. This means they have a special coating which allows the active ingredient, metformin, to be released slowly over time.
When to take METEX XR
Take your medicine everyday with the evening meal. Taking METEX XR during or with your evening meal will reduce the chance of a stomach upset. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take METEX XR for
Keep taking METEX XR for as long as your doctor recommends.
METEX XR will help control diabetes but will not cure it. Most people will need to take METEX XR for long periods of time.
When you start treatment with METEX XR, it can take up to some weeks for your blood glucose levels to be properly controlled.
If you forget to take METEX XR
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much METEX XR (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much METEX XR. Do this even if there are no signs of discomfort or poisoning. If you take too much METEX XR, you may feel very tired, sick, vomit, have trouble breathing and have unusual muscle pain, stomach pain or diarrhoea. These may be early signs of a serious condition called lactic acidosis (build up of lactic acid in the blood).
You may also experience symptoms of hypoglycaemia (low blood glucose). This usually only happens if you take too much METEX XR together with other medicines for diabetes or with alcohol.
If you do experience any signs of hypoglycaemia, raise your blood glucose quickly by eating jelly beans, sugar or honey, drinking non-diet soft drink or taking glucose tablets.
While you are taking METEX XR
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking METEX XR.
Tell all the other doctors, dentists and pharmacists who are treating you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor.
Tell your doctor if you plan to have:
- surgery with general anaesthesia
- any x-ray procedures requiring an injection of an iodinated contrast agent (dye).
Your doctor will advise you when to stop taking METEX XR before you have these procedures and when to start again.
HYPOGLYCAEMIA
METEX XR does not normally cause hypoglycaemia (low blood sugar) although you may experience it if you are also taking other medicines for diabetes such as insulin, sulfonylureas or glinide.
Make sure that you, your friends, family and work colleagues can recognise the symptoms of hypoglycaemia (low blood sugar) and know how to treat them
Hypoglycaemia can occur suddenly. Initial signs may include:
- weakness, trembling or shaking
- sweating
- light-headedness, dizziness, headache or lack of concentration
- irritability, tearfulness or crying
- hunger
- numbness around the lips and tongue.
If not treated promptly, these may progress to:
- loss of co-ordination
- slurred speech
- confusion
- fits or loss of consciousness.
If you experience any of the symptoms of hypoglycaemia, you need to raise your blood glucose immediately.
You can do this by doing one of the following:
- eating 5 to 7 jelly beans
- eating 3 teaspoons of sugar or honey
- drinking half a can of non-diet soft drink
- taking 2 to 3 concentrated glucose tablets.
Unless you are 10 to 15 minutes of your next meal or snack, follow up with extra carbohydrates such as plain biscuits, fruit or milk. Taking this extra carbohydrate will prevent a second drop in your blood glucose level.
HYPERGLYCAEMIA
If you experience any of the signs of hyperglycaemia (high blood sugar), contact your doctor immediately.
The risk of hyperglycaemia is increased in the following situations:
- uncontrolled diabetes
- illness, infection or stress
- taking less METEX XR than prescribed
- taking certain other medicines
- too little exercise
- eating more carbohydrates than normal.
Tell your doctor if any of the following happen:
- you become ill
- you become dehydrated (for instance due to persistent or severe diarrhoea or recurrent vomiting)
- you are injured
- you have a fever
- you have a serious infection such as an influenza, respiratory tract infection or urinary tract infection
- you are having major surgery
- you are having an examination such as an X-ray or a scan requiring injection of iodinated contrast agents (dye)
- you become pregnant.
Your blood glucose may become difficult to control in these situations. You may also be more at risk of developing a serious condition called lactic acidosis. In these situations, your doctor may replace METEX XR with insulin.
Visit your doctor regularly for check-ups. Your doctor may want to check your kidneys, liver, heart and blood while you are taking METEX XR.
Make sure you check your blood glucose levels regularly. This is the best way to tell if your diabetes is being controlled properly. Your doctor or diabetes educator will show you how and when to do this.
Carefully follow the advice of your doctor and dietician on diet, drinking alcohol and exercise.
Things you must not do
Do not use METEX XR to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not skip meals while taking METEX XR.
Do not stop taking your medicine or change the dose without checking with your doctor.
Things to be careful of
If you have to be alert, for example when driving, be especially careful not to let your blood glucose levels fall too low. Low blood glucose levels may slow your reaction time and affect your ability to drive or operate machinery. Drinking alcohol can make this worse. However, METEX XR by itself is unlikely to affect how you drive or operate machinery.
Things to be aware of
After metformin is absorbed into your body, you may see the empty tablet shell in your faeces (bowel motions). This is normal and does not affect the way metformin works
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking METEX XR.
METEX XR helps most people with diabetes but it may have unwanted side effects in some people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- stomach upset such as feeling sick (nausea), vomiting
- diarrhoea
- stomach pain
- taste disturbance, loss of appetite
- skin reactions such as redness of the skin, itching or any itchy rash (urticarial).
These are generally mild side effects which disappear after the first few weeks. Taking METEX XR with meals can help reduce nausea and diarrhoea.
Tell your doctor as soon as possible if you notice any of the following:
Symptoms of liver disease such as nausea, vomiting, loss of appetite, feeling generally unwell, fever, yellowing of the skin and eyes (jaundice) and dark coloured urine.
TELL YOUR DOCTOR IMMEDIATELY OR GO TO ACCIDENT AND EMERGENCY AT THE NEAREST HOSPITAL IF YOU NOTICE ANY OF THE FOLLOWING SYMPTOMS OF LACTIC ACIDOSIS (BUILD UP OF LACTIC ACID IN THE BLOOD):
- nausea, vomiting, stomach pain
- trouble breathing
- feeling weak, tired or generally unwell
- unusual muscle pain
- sleepiness
- dizziness or light-headedness
- shivering, feeling extremely cold
- slow heart beat
LACTIC ACIDOSIS IS A VERY RARE, BUT SERIOUS SIDE EFFECT REQUIRING URGENT MEDICAL ATTENTION OR HOSPITALISATION. ALTHOUGH RARE, IF LACTIC ACIDOSIS DOES OCCUR, IT CAN BE FATAL. THE RISK OF LACTIC ACIDOSIS IS HIGHER IN THE ELDERLY, THOSE WHOSE DIABETES IS POORLY CONTROLLED, THOSE WITH PROLONGED FASTING, THOSE WITH CERTAIN HEART CONDITIONS, THOSE WHO DRINK ALCOHOL AND THOSE WITH SEVERE KIDNEY OR LIVER PROBLEMS.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell. It is very important that you speak to your doctor immediately if a side effect is severe, occurred suddenly or gets worse rapidly.
Other side effects not listed above may also occur in some people. Some side effects (e.g. reduced vitamin B12 level) can only be found when your doctor does tests from time to time to check your progress.
After taking METEX XR
Storage
Keep METEX XR where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
Keep your METEX XR in a cool dry place where the temperature stays below 25°C.
Do not store METEX XR or any other medicine in the bathroom or near a sink.
Do not leave METEX XR in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking this medicine, or your tablets have passed their expiry date, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
METEX XR is available in 2 strengths of tablet:
- METEX XR 500mg – White, capsule shaped tablet, embossed with XR 500 on one side. Blister packs of 90* tablets and 120 tablets.
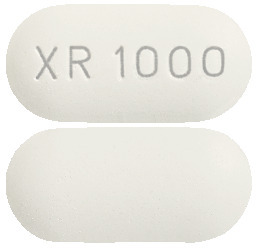
- METEX XR 1000mg – White, capsule shaped tablet, embossed with XR 1000 on one side. Blister packs of 60 tablets.
Ingredients
The active ingredient in METEX XR is metformin hydrochloride.
- each modified release METEX XR 500 contains 500 mg of metformin hydrochloride
- each modified release METEX XR 1000 contains 1000 mg of metformin hydrochloride.
The tablets also contain the following inactive ingredients:
- magnesium stearate
- colloidal anhydrous silica
- povidone
- hypromellose
The tablets do not contain sucrose, lactose, gluten, tartarazine or any other azo dyes.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
Australia
Australian registration numbers:
METEX XR 500mg - :
AUST R 232661
METEX XR 1000mg - :
AUST R 232645
Date of preparation:
December 2022.
Published by MIMS February 2023




 The prospective randomised (UKPDS) study has established the long-term benefit of intensive blood glucose control in overweight type 2 diabetes. The immediate release tablet form of metformin was used in the UKPDS.
The prospective randomised (UKPDS) study has established the long-term benefit of intensive blood glucose control in overweight type 2 diabetes. The immediate release tablet form of metformin was used in the UKPDS. Metformin modified release 750 mg tablet was shown to be bioequivalent to metformin modified release 500 mg tablet at a 1500 mg dose with respect to Cmax and AUC in healthy fed and fasted subjects.
Metformin modified release 750 mg tablet was shown to be bioequivalent to metformin modified release 500 mg tablet at a 1500 mg dose with respect to Cmax and AUC in healthy fed and fasted subjects. Molecular formula: C4H12ClN5. Molecular weight: 165.63.
Molecular formula: C4H12ClN5. Molecular weight: 165.63.