What is this medicine?
The name of this medicine is Movicol-Half. Each sachet contains:
- Macrogol 3350 - 6.536 g
- Sodium chloride - 175.4 mg
- Sodium bicarbonate - 89.3 mg
- Potassium chloride - 23.3 mg
When it is made into a drink with 62.5 mL of water, each sachet gives the equivalent of:
- Sodium - 65 mmol/L
- Chloride - 53 mmol/L
- Bicarbonate - 17 mmol/L
- Potassium - 5.4 mmol/L
Movicol-Half also contains lime and lemon flavour, and potassium acesulfame as a sweetener.
There are 30 sachets in a box. Each sachet contains 6.9 grams of Movicol-Half powder.
You add water to the powder to make a drink.
What is Movicol-Half used for?
Movicol-Half is used in adults and children aged 2 years and older for the treatment of chronic constipation. Constipation is the less frequent than normal passing of large, firm or hard stools. Movicol-Half helps you to have a comfortable bowel movement even if you have been constipated for a long time.
Chronic Constipation in Children:
Most normal children will occasionally experience constipation, which will normally require no more than a healthy diet, plenty of exercise, regular toilet use and, sometimes, occasional use of laxatives.
However, a small proportion of children will pass stools less frequently than 3 times per week, with excessive straining and discomfort or pain at these times. If your child has this more severe type of constipation your doctor needs to be involved in making a supervised plan of treatment for your child over the next 6 -12 months. Treatments may require daily use of a product such as Movicol-Half which can keep stools of normal consistency and restore a normal pattern of passing stools.
Movicol-Half is also used in children aged 2 years and older for the treatment of faecal impaction (as determined by your doctor), and for the prevention of recurrence of faecal impaction.
Before you take Movicol-Half
Do not take Movicol-Half if your doctor has told you that you have:
- an obstruction in your intestine (gut)
- a perforated gut wall
- severe inflammatory bowel disease, like ulcerative colitis, Crohn’s disease, or toxic megacolon
- paralysis of the bowel
- an allergy to macrogol or any of the ingredients.
Pregnancy and breast feeding
If you are pregnant, talk to your doctor before you take Movicol-Half.
Movicol-Half can be taken whilst breast feeding.
Other Medications
Laxative products such as Movicol-Half have the potential to interact with other medications, by altering their absorption. Close monitoring of the effects of your medications may be necessary when you commence or cease to take Movicol-Half regularly. It is important that you should discuss this with your doctor.
How to take Movicol-Half
Chronic Constipation/Prevention of recurrence of faecal impaction:
Children aged 2-5 years: The usual starting dose is 1 sachet daily.
Children aged 6-11 years: The usual starting dose is 2 sachets daily. The dose should be adjusted up or down as required to produce regular soft stools. The maximum dose needed does not normally exceed 4 sachets daily.
Use in children aged 2 years and older should be limited to 12 weeks except under medical supervision.
Movicol-Half is not recommended for children below 2 years of age.
Adults and children 12 years and older: The recommended dose of Movicol-Half is 2 sachets a day. This can be increased up to 6 sachets daily if necessary, depending on the severity of your constipation. For chronic constipation the dose may be reduced to 1 sachet daily, according to individual response.
For patients 12 years and older using 2 sachets daily or more, it is recommended to use Movicol.
FAECAL IMPACTION:
Children (2 - 11 years):
A course of treatment with Movicol-Half is for up to 7 days as follows:
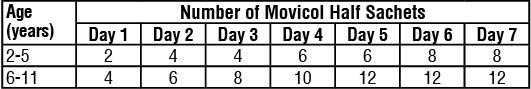
Treatment can be stopped when the medicine has worked. This is shown by the patient passing large volumes of stool or watery diarrhoea. It is not recommended to use Movicol-Half for the treatment of faecal impaction in children with a heart or kidney condition.
Open the sachet and pour contents into a cup. Add about 60 mL of water into the cup. Stir well until all the powder has dissolved and the Movicol-Half solution is clear or slightly hazy, then drink it. If you like, you can add a flavouring agent such as cordial to the drink.
For use in faecal impaction, the correct number of sachets can be made up in advance and kept covered and refrigerated for 24 hours. For example 12 sachets can be made up into 750 mL of water and 16 sachets into one litre of water.
Drink plenty of water and increase fibre in diet, except in cases of medication-induced constipation. Prolonged use of laxatives is undesirable and may lead to dependence. In some circumstances, prolonged use may be necessary but should only be under medical supervision.
Movicol-Half should not be used for the treatment of faecal impaction in children for longer than 7 days. If symptoms persist, see your doctor.
If you are taking Movicol-Half for constipation and get bad diarrhoea, stop taking Movicol-Half until it clears. If constipation recurs, you should check with your doctor or pharmacist before taking a new course of Movicol-Half. If you are worried, contact your doctor or pharmacist.
What about side effects?
Sometimes people have stomach ache or rumbles, or an allergic reaction, or feel bloated or sick. You may get diarrhoea, especially when you first start taking Movicol-Half. If you feel weak, breathless, very thirsty with a headache, or get puffy ankles stop taking Movicol-Half and tell your doctor. Tell your doctor or pharmacist if you think Movicol-Half is causing you any problem.
How to store Movicol-Half
Store your Movicol-Half at room temperature (below 25ºC). Do not use Movicol-Half after the expiry date on the label. Once you have made up Movicol-Half in water put it in the fridge (2-8ºC) and keep it covered. Throw away any solution not used within a 24 hour period. Keep all medicines away from children.
AUST R 109758
Date: 1 April 2015
Norgine Pty Limited,
3/14 Rodborough Road,
Frenchs Forest NSW 2086
1800 766 936
MOVICOL is a registered trademarks of the Norgine group of companies.

 The dosage regimen in Table 1 should be stopped once disimpaction has occurred. An indicator of disimpaction is the passage of a large volume of stools. After disimpaction, it is recommended that the child follows an appropriate bowel management program to prevent reimpaction.
The dosage regimen in Table 1 should be stopped once disimpaction has occurred. An indicator of disimpaction is the passage of a large volume of stools. After disimpaction, it is recommended that the child follows an appropriate bowel management program to prevent reimpaction.