SUMMARY CMI
Panamax Co
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
WARNING: Important safety information is provided in a boxed warning in the full CMI. Read before using this medicine.
1. Why am I using Panamax Co?
Panamax Co contains the active ingredients paracetamol and codeine phosphate hemihydrate. Panamax Co is used to relieve moderate pain. For more information, see Section 1. Why am I using Panamax Co? in the full CMI.
2. What should I know before I use Panamax Co?
Do not use if you have ever had an allergic reaction to Panamax Co or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Panamax Co? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Panamax Co and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Panamax Co?
The usual dose for Adults is 1 to 2 tablets. This dosage may be repeated every 4 to 6 hours if necessary
More instructions can be found in Section 4. How do I use Panamax Co? in the full CMI.
5. What should I know while using Panamax Co?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Panamax Co? in the full CMI.
6. Are there any side effects?
- Tell your doctor or pharmacist immediately if you notice any of the following side effects shortness of breath, wheezing or difficulty breathing, swelling of the face, lips, tongue, or other parts of the body, rash, itching or hives on the skin. They may be the signs of an allergic reaction.
- Tell your doctor or pharmacist if you notice any of the following and they worry you: nausea or vomiting; drowsiness or dizziness; constipation; stomach pain; skin rashes; sweating. These are the more common side effects of your medicine. They are usually mild.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
WARNING:
Limitations of use
Panamax Co should only be used if your doctor decides other treatment options are not able to effectively manage your pain or you cannot tolerate them.
Hazardous and harmful use
Panamax Co contains codeine which may be habit forming. Panamax Co poses risks of abuse, misuse and addiction which can lead to overdose and death. Your doctor will assess your risks and monitor you regularly during treatment.
Life threatening respiratory depression
Serious, life-threatening, or fatal respiratory depression (shallow or difficulty breathing) may occur with the use of Panamax Co even when used as recommended. These problems can occur at any time during use, but the risk is higher when first starting Panamax Co and after a dose increase, if you are older, or have an existing problem with your lungs. Your doctor will monitor you and change the dose as appropriate.
Use of other medicines while using Panamax Co
Using Panamax Co with other medicines that can make you feel drowsy such as sleeping tablets (e.g. benzodiazepines), other pain relievers, antihistamines, antidepressants, antipsychotics, gabapentinoids (e.g. gabapentin and pregabalin), cannabis and alcohol may result in severe drowsiness, decreased awareness, breathing problems, coma and death. Your doctor will minimize the dose and duration of use and monitor you regularly for signs and symptoms of breathing difficulties and sedation. You must not drink alcohol while taking Panamax Co.
FULL CMI
Panamax Co
Active ingredient(s): paracetamol and codeine phosphate hemihydrate
Consumer Medicine Information (CMI)
This leaflet provides important information about using Panamax Co. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Panamax Co.
Where to find information in this leaflet:
1. Why am I using Panamax Co?
2. What should I know before I use Panamax Co?
3. What if I am taking other medicines?
4. How do I use Panamax Co?
5. What should I know while using Panamax Co?
6. Are there any side effects?
7. Product details
1. Why am I using Panamax Co?
Panamax Co contains the active ingredients paracetamol and codeine phosphate hemihydrate.
Paracetamol and codeine work together to stop the pain messages from getting through to the brain. Your doctor may have prescribed this medicine for another use.
Panamax Co is a type of analgesic intended for short term use to relieve moderate pain.
2. What should I know before I use Panamax Co?
Warnings
Do not use Panamax Co if:
- you are allergic to paracetamol or codeine phosphate hemihydrate, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- you have severe and/or acute respiratory diseases
- you have acute breathing difficulties such as bronchitis, unstable asthma, emphysema (serious lung disease), respiratory depression (shallow breathing) or respiratory insufficiency (difficulty breathing).
- you have Glucose-6-phosphatedehydrogenase deficiency (an enzyme deficiency)
- you are an ultra-rapid metaboliser of CYP 2D6
- you have diarrhoea caused by antibiotics or poisoning
- you have chronic constipation
- you have liver failure
Do not take codeine if you have a history of drug dependence, including alcohol dependence.
Do not give Panamax Co to children under 12 years.
Do not give Panamax Co to children aged between 12-18 years who have undergone tonsillectomy and/or adenoidectomy to treat sleep apnoea.
Do not take Panamax Co during the third trimester of pregnancy.
Do not take codeine during labour, especially if the baby is premature.
Do not take it if you are breastfeeding or planning to breastfeed.
Do not take this medicine after the expiry date (EXP) printed on the pack.
If you take it after the expiry has passed, it may not work as well.
Do not take this medicine if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
Check with your doctor or pharmacist if you have or have had any of the following medical conditions:
- difficulty breathing, wheezing, chronic cough, asthma, or other chronic breathing conditions
- compromised respiratory function (due to emphysema, kyphoscoliosis or obesity)
- known analgesic intolerance
- a history of drug dependence, including alcohol dependence
- pre-existing opioid dependence
- chronic alcohol use including recent cessation of alcohol intake
- low glutathione reserves
- Gilbert's syndrome
- recent surgery on the stomach, intestine or urinary tract
- chronic constipation
- head injury or trauma
- prostate problems
- heart, liver or kidney problems
- urinary, bowel or gallbladder conditions
- Addison's disease
- Multiple sclerosis
- low blood pressure
- underactive thyroid
- convulsions, fits or seizures
Tell your doctor or pharmacist if you have allergies to aspirin, other NSAIDs, any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist if you plan to have surgery.
Tell your doctor or pharmacist if you take sedatives (medicines used to help you relax or sleep).
If you are not sure whether you should start taking this medicine, talk to your doctor or pharmacist.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor or pharmacist if you are pregnant or intend to become pregnant.
Do not take Panamax Co during the third trimester of pregnancy.
Do not take codeine during labour, especially if the baby is premature.
This medicine may produce withdrawal effects in the newborn baby.
Do not take it if you are breastfeeding or planning to breastfeed.
Panamax Co passes into breast milk and there is a possibility your baby may be affected.
If you have not told your doctor or pharmacist about any of the above, tell them before start taking Panamax Co.
Addiction
You can become addicted to Panamax Co even if you take it exactly as prescribed. Panamax Co may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking Panamax Co. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking Panamax Co suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to Panamax Co may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Panamax Co given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and Panamax Co may interfere with each other. These include:
- medicines used to help you relax, sleep or relieve anxiety, such as barbiturates, sedatives, tranquillisers, hypnotics, gabapentinoids, cannabis and centrally-active anti-emetics
- benzodiazepines (medicines used as sedatives or to treat anxiety)
- medicines containing alcohol (ethanol), e.g. some cough syrups
- antihistamines (medicines used to treat allergies)
- medicines which thin the blood
- medicines to treat epilepsy or fits
- metoclopramide or domperidone, medicines used to control nausea and vomiting
- propantheline, a drug used to relieve stomach cramps or spasms
- medicines used to prevent travel sickness and to treat Parkinson's disease
- medicines used to treat high blood pressure
- medicines for diarrhoea, such as kaolin, pectin, and loperamide
- medicines used to treat depression
- monoamine oxidase inhibitors, medicines used to treat depression, taken within the last 14 days
- other opioid analgesics used to treat pain
- quinidine, a medicine used to treat abnormal or irregular heartbeat
- phenothiazines and antipsychotic agents, medicines used to treat mental disorders
- chloramphenicol, an antibiotic used to treat ear and eye infections
- flucloxacillin, zidovudine or rifampicin, drugs used to treat infections
- chelating resin
- medicines used to treat alcohol and/or opioid dependence (e.g. naltrexone, buprenorphine or methadone)
- medicines used to control electrolytes levels in kidney disease
These medicines may be affected by Panamax Co or may affect how well Panamax Co works. You may need different amounts of your medicines, or you may need to take different medicines.
If you have not told your doctor or pharmacist about any of these things, tell him/her before you take any Panamax Co.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Panamax Co.
4. How do I use Panamax Co?
How much to take
- The label on your pack of Panamax Co will tell you how to take your medicine and how often.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
- The usual dose of Panamax Co is:
Adults:
- 1 to 2 tablets.
- This dosage may be repeated every 4 - 6 hours if necessary.
You should not take more than 8 tablets in 24 hours.
Do not take more than the recommended dose.
Talk to your doctor or pharmacist about pain control if Panamax Co is not helping.
If your body cannot metabolise codeine properly, you may be getting reduced benefit from the medicine.
If you are over 65 years of age, talk to your doctor or pharmacist about how much to take.
Elderly patients are more likely to have less effective kidney function due to age. This may increase the risk of side effects.
How to take Panamax Co
- Swallow tablets with a little water or other liquid.
How long to take it
Adults:
Only take Panamax Co for a few days at a time unless your doctor tells you to take it longer.
Children:
Only give Panamax Co to children for up to 48 hours unless a doctor has told you to give it longer.
If you take too much Panamax Co
If you or someone else receive too much (overdose), and experience one or more of the symptoms below, immediately call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally used Panamax Co that was prescribed for you. If someone takes an overdose they may experience one or more of the following symptoms:
- Slow, unusual or difficult breathing
- Drowsiness, dizziness or unconsciousness
- Slow or weak heartbeat
- Nausea or vomiting
- Convulsions or fits
If you think that you have taken too much Panamax Co, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
If you take too many tablets you may feel nauseous, experience stomach pain, sweating, anxiety, lightheaded, dizzy or drowsy for example.
You should do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this leaflet and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
Depending on your body's individual ability to break down codeine, you may experience signs of overdose even when you take Panamax Co as recommended by your doctor. If overdose symptoms occur, seek immediate medical advice.
5. What should I know while using Panamax Co?
Things you should do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking Panamax Co.
Talk to your doctor or pharmacist if your symptoms don't improve.
Your pharmacist or doctor will assess your condition and decide if you should continue to take the medicine.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking Panamax Co.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
Things you should not do
- Children: Do not give Panamax Co for more than 48 hours unless a doctor has told you to.
- Adults: Do not take for more than a few days at a time unless your doctor tells you to.
- Do not take more than the recommended dose unless your doctor tells you to.
- Do not take Panamax Co to treat any other complaints unless your doctor or pharmacist tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful if you are elderly, unwell or taking other medicines.
Some people may experience side effects such as drowsiness, confusion, dizziness and unsteadiness, which may increase the risk of a fall.
Driving or using machines
Be careful driving or operating machinery until you know how this medicine affects you.
This medicine may cause dizziness or light-headedness in some people
Children should be supervised while bike riding or engaging in other potentially hazardous activities to avoid potential harm.
Drinking alcohol
Do not drink alcohol while taking Panamax Co.
Drinking alcohol while taking paracetamol (which is contained in Panamax Co) may increase the risk of liver side effects.
Looking after your medicine
- Store below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Gastrointestinal related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Allergy related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Panamax Co contains
| Active ingredients (main ingredient) | Paracetamol and codeine phosphate hemihydrate. |
| Other ingredients (inactive ingredients) | Maize starch, povidone, potassium sorbate, microcrystalline cellulose, stearic acid, magnesium stearate, purified talc and pregelatinised maize starch. |
Do not take this medicine if you are allergic to any of these ingredients.
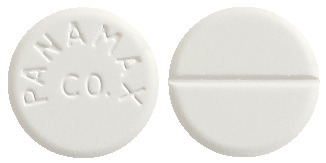
What Panamax Co looks like
Panamax Co is available as white tablets marked ‘PANAMAX CO’ (Aust R 62661).
Panamax Co is available in packs of 10 or 40 tablets.
*Not all pack sizes may be marketed
Who distributes Panamax Co
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park NSW 2113
AUSTRALIA
Toll Free Number (medical information): 1800 818 806
Email: [email protected]
This leaflet was prepared in December 2021.
panamax-co-ccdsv5-cmiv17-03dec21
Published by MIMS February 2022


 Paracetamol MW: 151.17. Codeine phosphate hemihydrate MW: 406.37.
Paracetamol MW: 151.17. Codeine phosphate hemihydrate MW: 406.37.