What is in this leaflet
This leaflet answers some common questions about PAXAM. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking PAXAM against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What PAXAM is used for
PAXAM is used to treat epilepsy in adults and children aged 2 years and over.
PAXAM contains the active ingredient clonazepam, which belongs to a group of medicines called benzodiazepines. These medicines are thought to work by their action on brain chemicals.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
The use of benzodiazepines may lead to dependence on the medicine. If you have any concerns, you should discuss this with your doctor.
This medicine is available only with a doctor's prescription.
Before you take PAXAM
When you must not take it
Do not take PAXAM if you have an allergy to:
- any medicine containing clonazepam or other benzodiazepines
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take PAXAM if you have:
- severe and chronic lung disease
- severe liver disease
- an addiction to drugs or alcohol
Do not take this medicine if you have galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption. People with these rare hereditary problems should not take this medicine as it contains lactose.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, dyes or preservatives.
Tell your doctor if you have or have had any of the following medical conditions:
- liver problems
- kidney problems
- lung problems
- high or low blood pressure
- glaucoma, a condition characterised by an increased pressure in the eye
- myasthenia gravis, a condition characterised by severe muscle weakness
- depression, psychosis, schizophrenia
- spinal or cerebellar ataxia, condition of clumsiness or in coordination of the muscles
- history of addiction or drug dependence
- porphyria, a rare hereditary disorder which affects blood pigment
- sleep apnoea, a condition where you stop breathing when you're asleep; PAXAM is not recommended for use in patients with sleep apnoea due to possible additive effects on respiratory depression.
Tell your doctor if you are pregnant or plan to become pregnant. It is not known whether PAXAM is safe to use during pregnancy. There have been reports of unwanted effects occurring in the newborn with the use of medicines of this class when used during pregnancy.
Your doctor will discuss with you the risks and benefits involved.
Tell your doctor if you are breastfeeding or plan to breastfeed. The active ingredient in PAXAM passes into breast milk and may cause drowsiness and feeding difficulties in the baby.
Tell your doctor if you drink alcohol. Alcohol may change the effects of PAXAM and may even cause you to have an epileptic seizure.
If you have not told your doctor about any of the above, tell them before you start taking PAXAM.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and PAXAM may interfere with each other. These include:
- other medicines for epilepsy, such as phenytoin, carbamazepine, sodium valproate
- sleeping tablets, sedatives, muscle relaxants
- medicines for depression such as tricyclic antidepressants, monoamine oxidase inhibitors
- medicines for mental illness
- antihistamines, medicines for allergies or colds
- pain relievers
- anaesthetics
- cimetidine, a medicine used to treat reflux and stomach ulcers
- disulfiram, a medicine used to deter alcohol consumption
- lithium, a medicine used to treat mood swings and some types of depression.
- fluconazole, an antifungal medication used for a number of fungal infections
These medicines may be affected by PAXAM or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take PAXAM
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle, ask your doctor or pharmacist for help.
How much to take
Take PAXAM exactly as directed by your doctor. Your doctor will tell you how many tablets you need to take each day and when to take them. The dose may depend on your age, your medical condition and whether or not you are taking any other medicines.
PAXAM is usually started using a low dose. Your doctor may gradually increase this dose to the lowest amount needed to control your condition depending on how well you respond to and tolerate the medicine.
The usual adult maintenance dose is between 4 mg and 8 mg a day.
Children, the elderly and people with liver or kidney problems may need smaller doses.
How to take it
Swallow the tablets with a glass of water. PAXAM tablets can be broken in half or quarters if your doctor has prescribed half or quarter of a tablet.
When to take it
PAXAM is usually taken twice a day (in the morning and evening). However, depending on your dose, your doctor may recommend you take it three or four times a day.
PAXAM can be taken with or without food.
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
Continue taking your medicine for as long as your doctor recommends. This medicine helps to control your condition but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much PAXAM. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Symptoms of an overdose may include feeling drowsy, tired, confused, dizzy, having difficulty breathing, feeling weak or become unconscious.
While you are taking PAXAM
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking PAXAM.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, including dental surgery, tell the surgeon, anaesthetist or dentist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
Tell your doctor if you feel that this medicine is not helping your condition. If you continue to have seizures (fits) your doctor may need to adjust or review your treatment.
Tell your doctor if, for any reason, you have not taken PAXAM exactly as prescribed. Otherwise, your doctor may adjust your treatment unnecessarily.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may ask you to have regular blood tests to check your blood count, kidney and liver function.
Things you must not do
Do not take PAXAM to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. Stopping this medicine suddenly may make your epilepsy worse and cause some unwanted effects. Your doctor will tell you how to gradually reduce the amount of PAXAM you are taking before stopping completely.
Do not drink alcohol while you are taking PAXAM. Combining this medicine and alcohol can make you more sleepy or dizzy. Alcohol can also affect how well PAXAM works and may even cause more seizures (fits).
Be careful driving or operating machinery until you know how PAXAM affects you. This medicine may cause drowsiness, dizziness or affect alertness in some people.
These effects may continue the following day.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Children should not ride a bike, climb trees or do anything else that could be dangerous if they are feeling drowsy or sleepy.
Do not take Paxam for a longer time than your doctor has prescribed.
Things to be careful of
If PAXAM is being given to a young child, you should be especially careful that they are breathing freely.
PAXAM may increase the amount of saliva and fluid in the airways.
Be careful if you are elderly, unwell or taking other medicines. Some people may experience side effects such as drowsiness, confusion, dizziness and unsteadiness, which may increase the risk of a fall.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking PAXAM.
This medicine helps most people with their epilepsy, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- drowsiness, tiredness
- dizziness, light-headedness
- unsteadiness when walking
- muscle weakness
- slurred speech
- loss of memory, inattentiveness, confusion, lack of concentration, slowed reactions
- increased saliva
- chest congestion
- headaches, hangover feeling in the morning
- unpleasant dreams
The above list includes the more common side effects of your medicine. They may disappear with continued treatment.
Tell your doctor as soon as possible if you notice any of the following and they worry you:
- behaviour changes such as aggression, agitation, irritability, depression, restlessness, nervousness, hostility, anxiety, sudden feelings of rage
- severe sleep disturbances, nightmares, vivid dreams
- hallucinations or delusions
- bleeding or bruising more easily than normal
- signs of frequent infections such as fever, severe chills, sore throat, mouth ulcers.
The above list includes serious side effects which may require medical attention. Some of these side effects are rare.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at the nearest hospital:
- swelling of the face, lips, mouth, throat or neck, which may cause difficulty swallowing or breathing
- fainting
- more fits than usual
- difficulty breathing, shortness of breath
- chest pain
- thoughts of self-harm
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking PAXAM
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they will not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store PAXAM or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
PAXAM is available in 2 strengths:
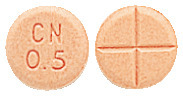
- PAXAM 0.5 - round, peach coloured tablet marked "CN" over "0.5" on one side and cross scored on the other.
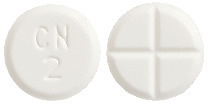
- PAXAM 2 - round, white tablet marked "CN" over "2" on one side and cross scored on the other.
Each bottle contains 100 tablets.
Ingredients
PAXAM contains either 0.5 mg or 2 mg of clonazepam as the active ingredient.
The tablets also contain the following inactive ingredients:
- lactose monohydrate
- maize starch
- microcrystalline cellulose
- magnesium stearate
- sunset yellow FCF aluminium lake [PAXAM 0.5 tablet only].
PAXAM also contains galactose and sulfites.
PAXAM tablets do not contain gluten.
Manufacturer
Alphapharm Pty Limited
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration numbers:
PAXAM 0.5 - AUST R 54846
PAXAM 2 - AUST R 54847
This leaflet was prepared in July 2023.
paxam_cmi\Jul23/00
Published by MIMS September 2023

 The daily quota should, if possible, be divided into three or four doses spread over the day.
The daily quota should, if possible, be divided into three or four doses spread over the day. Other adverse events which have been reported and may be related to clonazepam administration are presented in Table 3.
Other adverse events which have been reported and may be related to clonazepam administration are presented in Table 3.
 Clonazepam is a light yellow powder which is practically insoluble in water.
Clonazepam is a light yellow powder which is practically insoluble in water.