What is in this leaflet?
This leaflet answers some common questions about Riamet Dispersible tablets. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. Some more recent information on the medicine may be available.
You should speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most current leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of your child taking this medicine against the benefits they expect it will provide.
If you have any concerns about this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Riamet Dispersible is used for
Riamet Dispersible tablets contain two antimalarial medicines, artemether and lumefantrine. These ingredients work together to kill the Plasmodium falciparum parasite in uncomplicated or mixed infections of malaria.
Malaria commonly occurs in sub-tropical and tropical areas. Riamet Dispersible is used to treat malaria infections acquired in areas where malaria parasites may be resistant to other anti-malarial medicines.
Malaria is an infectious mosquito-borne disease, spread to humans by the bite of the Anopheles mosquito. The mosquito carries parasites and injects them into the bloodstream when it bites a person.
The parasites infect red blood cells, causing fever, chills, a general feeling of unwell (malaise), cough, nausea, headaches, vomiting and diarrhoea. Not all symptoms need to be present to suggest that your child has malaria.
Ask your doctor if you have any questions about why this medicine has been prescribed for your child. Your doctor may have prescribed it for another purpose.
This medicine is only available with a doctor's prescription. It is not addictive.
This medicine is suitable for infants and children that:
- weigh between 5 kg and less than 35 kg
- are aged between 3 months and under 12 years of age.
It is not suitable for any child or infant that weighs less than 5 kg or is less than 3 months in age.
Before you give it to your child
When you must not give it
Do not give your child Riamet Dispersible tablets:
- to prevent your child getting malaria
- to treat severe malaria (e.g. affecting your child's brain, kidneys, or lungs)
- if your child is in the first trimester of pregnancy and/or intends to become pregnant and if it is possible for the doctor to give an alternative malaria medicine
- if your child is breast feeding.
Tell your doctor if your child has, or has ever had, any of the following:
- severe liver or kidney problems
- a heart condition, such as:
- changes in the rhythm or rate of the heart beat
- slow heart beat
- severe cardiac disease
- abnormal electrical signal called "prolongation of the QT interval"
- any other heart problem - a family history of heart rhythm problems or sudden death (i.e. parents, grandparents, brothers and sisters who have died suddenly due to a heart rate problem or is known to have been born with heart rate problems.)
- treatment with medicines that affect the heart beat (known as anti-arrhythmics)
- low blood electrolytes levels of potassium or magnesium
- an allergic (hypersensitivity) reaction to artemether, lumefantrine, or to any of the other ingredients listed at the end of this leaflet.
Tell your doctor if your child is allergic to any other medicines, foods, dyes or preservatives.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Your doctor will want to know if your child is prone to allergies.
Ask your doctor for advice if you think your child may be allergic.
Do not give Riamet Dispersible if your child is taking certain medicines to treat:
- an abnormal heart rhythm, heart rhythm disturbances (such as flecainide, metoprolol)
- depression or mental illness (such as imipramine, amitriptyline, clomipramine)
- infections using:
- rifampicin, an antibiotic used to treat leprosy or tuberculosis
- antibiotics - including medicines of the following classes: macrolides, fluoroquinolones, imidazole
- triazole antifungal agents (such as fluconazole, itraconazole) - allergies or inflammation (e.g. non-sedating antihistamines such as terfenadine or astemizole)
- slow heart beat or changes in heart beat rate
- hyperacidity, reflux, ulcers, or stomach disorders (e.g. cisapride)
- epilepsy (such as phenytoin, or carbamazepine)
- some temporary feelings of sadness or low mood with a medicinal plant extract called St John's wort (Hypericum perforatum)
Do not give Riamet Dispersible after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
Before you start to give it
Tell your doctor if your daughter is pregnant, thinks she may be pregnant, or if she becomes pregnant while taking Riamet Dispersible. This medicine may affect your child's developing baby especially if taken during the first three (3) months of pregnancy. There are potential serious consequences for the foetus. It may be possible for the doctor to give an alternative medicine during this time.
Only give Riamet Dispersible to your daughter in the later stages of her pregnancy if it is:
- clearly necessary and
- advised by your doctor.
Your doctor will discuss the potential risk of taking Riamet Dispersible during pregnancy with you and your daughter.
Adolescent women who are capable of becoming pregnant are advised to use an effective method of contraception while on Riamet Dispersible treatment, and until the start of the next menstruation after treatment.
If your daughter is taking hormonal birth control medicine, she should also use an additional method of birth control.
Taking other medicines
Some medicines and Riamet Dispersible may interfere with each other.
Tell your doctor or pharmacist if your child is taking or has recently taken:
- any other medicines to treat malaria
- any medicines used to treat HIV infections or AIDS (e.g. anti-retrovirals or protease inhibitors)
- hormonal birth control medication (as your child should follow an additional method of birth control whilst taking Riamet Dispersible)
- medicines that are removed from the body by the liver
- any other medicines, including any that you may have bought without a prescription from a pharmacy, supermarket or health food shop
Your child may need to take different amounts of these medicines or take different medicines. Your doctor and pharmacist will have more information.
If you have not told your doctor about any of these things, tell him/her before your child is given this medicine. It is very important that you give your doctor this information before your child starts to take Riamet Dispersible tablets.
How to give it to your child
Give your child Riamet Dispersible immediately after some food. Giving it with food increases the amount of medicine that is absorbed into the body. This helps to kill the malaria parasite more effectively and reduces the risk of a relapse infection (return of malaria).
How to give it to your child
- Immediately before giving Riamet Dispersible to your child, place the tablet(s) in a drinking glass or cup, containing a small amount of water (approximately 10 mL per tablet).
- Allow the tablet(s) to disintegrate and stir gently before giving the cherry flavoured solution to your child to drink.
- Afterwards, immediately rinse the vessel with an additional small amount of water (approximately 10 mL) and give it to your child to drink completely.
- The tablets should be immediately followed by food or drinks rich in fat (e.g. milk).
Contact your doctor or pharmacist immediately if your child vomits within one hour of taking the dose. You may need to give your child another dose.
If your child is too unwell to eat or drink, you should still give him/her Riamet Dispersible as prescribed. Children with malaria often do not feel like eating, but your child should try to eat normally as soon as they can tolerate food.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to give
The treatment consists of a six-dose regimen, given over 60 hours (2.5 days). The following recommended doses are based on your child's body weight:
- 5 kg to less than 15 kg: 1 tablet per dose
- 15 kg to less than 25 kg: 2 tablets per dose
- 25 kg to less than 35 kg: 3 tablets per dose.
The number of tablets per dose will be the same at each time interval. Your doctor or pharmacist will tell you how many Riamet Dispersible tablets to give your child.
Follow your doctor's instructions carefully, and do not exceed the recommended dose. Their directions may differ from the information contained in this leaflet.
Ask your doctor or pharmacist if you are unsure about how many Riamet Dispersible tablets to give.
When to give it
Start your child's treatment at the time of malaria diagnosis by a doctor.
Unless your doctor tells you otherwise, give your child the six doses (over 3 days) as follows:
- Dose 1: given at the time of initial malaria diagnosis
- Doses 2 to 6: given twice daily (morning and evening), with an interval of at least 8 hours after the previous dose
To benefit from the full therapeutic effect, the full course of medication must be given at the intervals indicated.
Ask your doctor or pharmacist if you are unsure when to give it to your child.
How long to give it
Your child should continue taking Riamet Dispersible for the full six-dose course of treatment recommended by your doctor (over 60 hours). It is extremely important to give this medicine to your child exactly as directed by your doctor and for the full course of treatment, even if your child begins to feel better before you have finished the tablets.
If your child stops taking Riamet Dispersible too soon, your child's symptoms may return.
Do not miss giving your child any doses. Falciparum malaria is a serious, life-threatening disease that requires complete cure.
If you forget to give it
Give your child the missed dose of Riamet Dispersible as soon as you realise that you have forgotten to give it. Then give the next dose at the usual time.
Do not give your child a double dose to make up for individual doses that you have forgotten. Your child's chance of an unwanted side effect may be increased if you do this.
If you have trouble remembering when to give your child the medicine, ask your pharmacist for some hints.
If you give your child too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital if you think that you have accidentally given your child too many Riamet Dispersible tablets. Your child may require medical attention.
Do this even if there are no signs of discomfort or poisoning.
Remember to take the medicine carton with you, and show it to your doctor or to the staff of the Accident and Emergency unit. If you have run out of tablets, take the empty packaging along with you.
Keep the telephone numbers for your doctor and these places handy.
While your child is taking it
Things you must do
Remember to give your child all six doses at the indicated time intervals.
Contact your doctor immediately if your child's condition worsens or if he/she feels too unwell to eat or drink. Your doctor may want to perform a test called an electrocardiogram (ECG) and check the levels of electrolytes such as potassium and magnesium in your child's blood before and during treatment.
Your daughter must not breast-feed whilst taking this medicine and for 4 weeks after she has taken the last tablet. It is not known if the active ingredient in Riamet Dispersible passes into the breast milk and could affect your child's baby.
Tell your doctor if your child does not feel like eating while taking Riamet Dispersible. People with malaria usually don't feel like eating. However, eating may help to stop the malaria coming back.
Tell your doctor if your child keeps vomiting. If this happens, the medicine may not work properly. Your doctor may need to give your child another treatment.
Tell your doctor if your child's symptoms are not improving, after starting Riamet Dispersible.
If your child is about to be started on any new medicine, remind your doctor and pharmacist that he/she is taking Riamet Dispersible tablets.
Tell any doctor, dentist or pharmacist who treats your child that they are taking Riamet Dispersible tablets.
Things you must not do
Do not use this medicine to treat any other complaints, unless your doctor tells you to.
Do not give this medicine to anyone else, besides your child, even if their condition seems similar to your child's.
Things to be careful of
Contact your doctor immediately if your child feels ill again, especially if he/she develops a fever after finishing treatment. A further course of treatment with Riamet Dispersible may be necessary if the malaria infection returns (i.e. your child has a relapse) or is reinfected with Plasmodium falciparum after having been cured.
Avoid giving your child grapefruit juice whilst they are taking this medicine.
Do not let your child do anything that could be dangerous, as this medicine may make some infants or children feel sleepy, dizzy or weak. Your child should be careful doing anything that requires him/her to be alert until you know how this medicine affects them.
Side effects
Tell your doctor or pharmacist as soon as possible if your child does not feel well whilst taking Riamet Dispersible. All medicines can have side effects. Sometimes they are serious, most of the time they are not. Your child may need medical treatment if he/she gets some of the side effects.
Your child may not be able to tell the difference between side effects of Riamet Dispersible and the symptoms of malaria itself.
Do not be alarmed by these lists of possible side effects. Your child may not experience any of them. Most of the side effects are mild to moderate and will generally disappear after a few days to a few weeks of treatment.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if your child has any of the following common side effects:
- headache
- dizziness
- loss of appetite
- stomach pain or problems
- nausea (feeling sick) or vomiting
- diarrhoea
- difficulty sleeping or sleepiness
- aching muscles or joints
- unsteadiness when walking
- tingling or numbness of the hands or feet
- sore throat
- cough
- fever
- shivering
- itching on the skin or a rash
Tell your doctor immediately if any of the following happen:
- sudden signs of allergy such as rash, itching or hives on the skin; swelling of the face, lips, tongue or other parts of the body;
- wheezing or troubled breathing
- unusual bleeding or bruising under the skin
- feeling of fast or irregular heart beat (palpitations)
- signs of a possible liver problem such as: unexplained persistent nausea, persistent pain in the upper right abdomen, yellowing of the skin and/or eyes, dark urine or pale bowel motions, unusual tiredness or general weakness
- lightheadedness, fainting or near fainting
- involuntary muscle contractions, sometimes in rapid spasms
Some side effects may not give your child any symptoms and can only be found when tests are done. Some of these side effects include:
- heart rhythm disturbances (called QTc prolongation or abnormal ECG heart tracing).
Tell your doctor if you notice anything else that is making your child feel unwell. Some infants or children may have other side effects not yet known or mentioned in this leaflet.
After giving Riamet Dispersible
If your child is infected with both Plasmodium falciparum and Plasmodium vivax, your doctor will prescribe another medicine for your child to take after completing Riamet Dispersible treatment.
Storage
- Keep your child's medicine in the original container until it is time to give a dose
- Store this medicine below 30°C in a dry place
- Do not store this or any other medicine in the bathroom or near a sink
- Do not leave it in the car or on window sills.
Keep this medicine where children cannot see or reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
Ask your pharmacist what to do with any medicine you have left over, if your doctor tells you to stop taking this medicine or if the expiry date has passed.
Product description
What it looks like
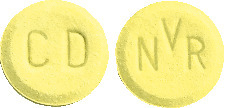
Riamet 20 mg/120 mg Dispersible tablets are round, flat, and yellow. "CD" is imprinted on one side and "NVR" on the other side.
Each carton contains 18 tablets.
Ingredients
Riamet Dispersible tablets contain:
- artemether 20 mg and
- lumefantrine 120 mg.
The tablets also contain:
- cellulose - microcrystalline (E 460)
- croscarmellose sodium
- hypromellose (E 464)
- magnesium stearate (E 572)
- polysorbate 80
- silica-colloidal anhydrous
- dry cherry flavour
- saccharin sodium (E 954)
Excipients with known effect: sugars, saccharin and latex (in trace amounts).
Riamet Dispersible tablets do not contain sucrose, lactose, gluten, tartrazine or any other azo dyes.
Sponsor
Riamet Dispersible tablets are supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone: 1800 671 203
Web site: www.novartis.com.au
®= Registered Trademark
This leaflet was prepared in June 2020.
Australian Registration Number:
Riamet 20 mg/120 mg Dispersible tablets: AUST R 158523
This leaflet (riaDT240620c) was prepared based on PI (ria240620i )
Published by MIMS September 2020

 Due to limitations of this analysis, the risk of adverse pregnancy outcomes for artemether-lumefantrine exposed women in early pregnancy cannot be excluded.
Due to limitations of this analysis, the risk of adverse pregnancy outcomes for artemether-lumefantrine exposed women in early pregnancy cannot be excluded. Adverse events found in nonrecommended regimens not included in this pooled safety analysis: paraesthesia (1.2% of adolescents and adults, no cases in children); involuntary muscle contractions (1.3% of children).
Adverse events found in nonrecommended regimens not included in this pooled safety analysis: paraesthesia (1.2% of adolescents and adults, no cases in children); involuntary muscle contractions (1.3% of children). Serious adverse events that occurred in patients taking Riamet in clinical trials included isolated reports of haemolytic anaemia, hepatitis and QTc prolongation. Asymptomatic QTc prolongation was reported in adults, children and infants but no causal relationship with Riamet could be confirmed.
Serious adverse events that occurred in patients taking Riamet in clinical trials included isolated reports of haemolytic anaemia, hepatitis and QTc prolongation. Asymptomatic QTc prolongation was reported in adults, children and infants but no causal relationship with Riamet could be confirmed.

 In an open, multicentre clinical study conducted in Africa in 310 children weighing ≥ 5 kg to ≤ 25 kg and receiving a 6-dose Riamet according to body weight ranges, the mean 28-day parasitological cure rate (PCR corrected) was 93.9% for the ITT population and 96.7% for the evaluable population.
In an open, multicentre clinical study conducted in Africa in 310 children weighing ≥ 5 kg to ≤ 25 kg and receiving a 6-dose Riamet according to body weight ranges, the mean 28-day parasitological cure rate (PCR corrected) was 93.9% for the ITT population and 96.7% for the evaluable population.



