What is in this leaflet
This leaflet answers some of the common questions about RISPERDAL. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
If you have any concerns about using RISPERDAL, ask your doctor or pharmacist. Your doctor and pharmacist have more information.
Keep this leaflet with your medicine. You may need to read it again.
What RISPERDAL is used for
RISPERDAL belongs to a group of medicines called antipsychotic agents which improve the symptoms of certain types of mental illness.
It is used for:
- treatment of sudden (acute) and long-term (chronic) schizophrenia and other types of related psychoses. These are disorders related to thought, feeling and/or action,
- short term treatment of acute mania associated with bipolar 1 disorder. This condition is characterised by symptoms such as elevated, expansive or irritable mood, inflated self-esteem, decreased need for sleep, pressured speech, racing thoughts, distractibility or poor judgement including disruptive or aggressive behaviours,
- treatment of behavioural problems in patients with a decline in mental ability (dementia) caused by Alzheimer's disease. These problems include: aggression through words or action, morbid suspiciousness, agitation or wandering,
- treatment of conduct and other disruptive behaviours such as aggression, impulsiveness and self-injury in children (over 5 years old), adolescents and adults who are intellectually disabled,
- treatment of behavioural symptoms of autism in children and adolescent.
RISPERDAL helps to correct a chemical imbalance in the brain associated with these conditions.
This medicine has been approved for the uses mentioned above. However, your doctor may prescribe it for another use. It is only available with a doctor's prescription.
If you want more information, ask your doctor.
RISPERDAL is not addictive.
Before you use RISPERDAL
When you must not use it
Do not use RISPERDAL:
- if you know you are allergic to any of its ingredients (see the last section of this leaflet for a list of ingredients)
Signs of allergy include skin rash, itching, shortness of breath, and/or swollen face - if the packaging is torn or shows signs of being tampered with
- if the tablets or the oral solution do not look right
- to treat any other complaints unless your doctor says it is safe to do so.
Before you start to use it
RISPERDAL should be used with caution in some patients.
- Tell your doctor if you have or have ever had any medical conditions, especially the following:
- irregular heart rhythm, abnormalities in electrical activity of the heart, high or low blood pressure, or you've had a heart attack or stroke in the past.
- unusual excessive sweating or diarrhoea, dehydration or problems with your body temperature regulation
- kidney or liver problems
- you are prone to dizziness when standing up from lying or sitting position
- Parkinson's disease (a progressive movement and thinking disorder that tends to affect older people)
- dementia or Lewy body dementia
Older people suffering dementia may be at increased risk of stroke or death with RISPERDAL - sugar diabetes
- unusual thirst, tiredness, blurred vision, upset stomach or need to urinate - common signs of high blood sugars
- epilepsy, seizures or fits
- continuous and/or painful erections (called 'priapism')
- involuntary movements or unusual restlessness or difficulty sitting still
- suicidal thoughts or past suicide attempts
- low blood potassium levels (hypokalaemia)
- breast cancer
- cancer of the pituitary gland
- Tardive dyskinesia (a reaction to some medicines with uncontrollable twitching or jerking movements of the tongue, face, mouth, jaw, arms and legs)
- Neuroleptic Malignant Syndrome (a serious reaction to some medicines that causes sudden increase in body temperature, very fast heartbeat, extremely high or low blood pressure and severe muscle stiffness or fits).
- blood clots
Tell your doctor if you or someone else in your family has a history of blood clots. Blood clots in the lungs and legs can occur with RISPERDAL. Blood clots in the lungs can result in death. - low white blood cell count
If you have low numbers of some white blood cells, your risk of contracting an infection or developing a fever is increased with RISPERDAL.
- Tell your doctor if:
- you have any eye surgery planned.
Your doctor will need to assess whether you are at risk of a surgical complication (called 'Intraoperative Floppy Iris Syndrome). You may be recommended to stop your RISPERDAL temporarily prior to your eye surgery. - you are pregnant or planning to become pregnant.
Your doctor will advise you whether you should take RISPERDAL.
Newborn babies of mother taking RISPERDAL in their last trimester may be at risk of having difficulty feeding or breathing, shaking, muscle stiffness and/or weakness, sleepiness or agitation. - you are breast-feeding.
As RISPERDAL is excreted in breast milk, it is recommended that you do not breast-feed while taking the medicine. - you will be in a hot environment or do a lot of vigorous exercise.
RISPERDAL may make you sweat less, causing your body to overheat.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking RISPERDAL.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
In particular, tell your doctor if you are taking:
- sleeping tablets, tranquillisers, strong pain-killers, certain allergy medicines called antihistamines, certain antidepressants and alcohol
- medicines that increase the activity of the central nervous system (psychostimulants such as methylphenidate).
- medicines used to treat bacterial infections such as rifampicin
- medicines to treat fungal infections such as itraconazole and ketoconazole
- medicines to treat HIV/AIDS, such as ritonavir and tipranavir
- other medicines to treat mental illness or psychotic conditions
- medicines to treat depression, panic disorder, anxiety or obsessive-compulsive disorder, such as fluoxetine, paroxetine, sertraline and fluvoxamine
- medicines for your heart or blood pressure
- verapamil, a medicine used to treat high blood pressure and/or abnormal heart rhythm
- furosemide, a medicine used to treat high blood pressure and fluid build-up.
There is an increased risk of side effects or death in older people if furosemide is also taken with RISPERDAL. - medicines to treat epilepsy
- carbamazepine, a drug mainly used for epilepsy or trigeminal neuralgia (severe pain attacks in the face)
- medicines to treat Parkinson's disease or a tremor.
Taking it for the first time.
At the start of treatment, you may have a fall in blood pressure making you feel dizzy on standing up, or your heart may beat faster. These should go away after a few days. Tell your doctor if they continue or worry you.
Using RISPERDAL
How much to take
Your doctor will decide the dose suitable for you.
Follow your doctor's instructions carefully and do not change or stop the required dosage without consulting your doctor first.
Important note: never take more tablets or solution than your doctor tells you to take.
RISPERDAL cannot be recommended for use in children with schizophrenia under 15 years at the present time as there is little experience with the product in this group.
For Schizophrenia and Related Psychoses
The usual starting dose of RISPERDAL is 1 mg twice a day. This will be gradually increased by your doctor to suit your needs.
From then on, the dose can be taken once a day or twice a day according to your doctor's instructions.
For Elderly Patients with Schizophrenia or Related Psychoses
For older patients a starting dose of 0.5 mg (or 0.5 mL of solution) twice a day (in the morning and in the evening is usual). This may be gradually increased by your doctor to suit your needs.
Patients with impaired kidney and liver function.
If you have kidney or liver disease a starting dose of 0.5 mg (or 0.5 mL of solution) twice a day (in the morning and in the evening) is usual. This may be gradually increased by your doctor to suit your needs.
For acute mania
The recommended starting dose is 2mg once a day. This may be gradually increased by your doctor to suit your needs.
Your doctor may decide you should take another medicine called a mood stabiliser as well as RISPERDAL.
For Behavioural Problems in People with Dementia
The usual starting dose is 0.25 mg twice daily. This may be gradually increased by your doctor to suit your needs.
For Disruptive Behaviour Disorders in Adults and Children
For people who weigh 50 kg or more, the usual starting dose is 0.5 mg (or 0.5 mL of solution) once a day. This may be gradually increased by your doctor to suit your needs.
For people who weigh less than 50 kg, the usual starting dose is 0.25 mg once a day. This may be gradually increased by your doctor to suit your needs.
Your doctor will advise you on how much RISPERDAL you need.
RISPERDAL cannot be recommended for use in children with disruptive behaviour disorders under 5 years at the present time as there is little experience with the product in this group.
For Behavioural Disorders Associated with Autism in Children and Adolescents
For people weighing less than 20kg the usual starting dose is 0.25mg. This may be gradually increased by your doctor to suit your needs.
For people weighing 20kg or more the usual starting dose is 0.5mg. This may be gradually increased by your doctor to suit your needs.
Your doctor will advise you on how much RISPERDAL you need.
When to take it
RISPERDAL may be taken as a single dose, once a day or it may be taken in divided doses twice a day (in the morning and in the evening). You may take RISPERDAL either with or between meals.
How to take it
RISPERDAL Tablets
Swallow RISPERDAL tablets with water or other liquid.
RISPERDAL oral solution
Mix RISPERDAL oral solution with a non-alcoholic drink.
Mineral water, orange juice, coffee and milk are suitable. Do not use tea.
It is very important that you take the correct amount of RISPERDAL, but this will vary from person to person. Your doctor will adjust the dose and strength of the tablets or the amount of solution until the desired effect is obtained.
How to use
Directions for opening the bottle and using the pipette for RISPERDAL Oral Solution
Step 1:
The bottle comes with a child-resistant cap.
To open, push the screw cap down while turning it anti-clockwise. Remove the unscrewed cap.
Step 2:
Use the pipette from the container. While holding the bottom ring, pull the top ring up to the mark that corresponds to the dose you need.
Step 3:
Holding the bottom ring, remove the entire pipette from the bottle.
Step 4:
Empty the contents of the pipette into a non-alcoholic drink by sliding the upper ring down. Mineral water, orange juice, coffee and milk are suitable. Do not use tea.
Step 5:
Close the bottle. Rinse the pipette with some cold water after use, dry and store it in its case. Use of detergents or extensive rubbing with a cloth may increase the risk of fading or disappearing print.
How long to take it
Continue taking the tablets or solution for as long as your doctor tells you.
RISPERDAL helps control your condition but does not cure it. Therefore, you must take RISPERDAL every day.
Do not stop taking it unless your doctor tells you to - even if you feel better.
If you forget to take RISPERDAL
- If you forget to take RISPERDAL, take the missed dose as soon as you remember instead of your next dose. Then go back to taking it as you would normally.
- Do not take a double dose to make up for the one you missed.
- If you forget to take RISPERDAL for a number of days or more, tell your doctor before starting your medicine again.
If you have problems remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much
Immediately telephone your doctor or the Poisons Information Centre or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much RISPERDAL. Do this even if there are no signs of discomfort or poisoning.
You can contact the Poisons Information Centre by dialling:
- Australia: 13 11 26
- New Zealand: 0800 POISON or 0800 764 766.
Signs of overdose may include drowsiness, sleepiness, excessive trembling, excessive muscle stiffness, increased heart rate, very low blood pressure causing fainting or unconsciousness.
While you are using RISPERDAL
Things you must do
Always follow your doctor's instructions carefully and seek your doctor's advice before changing or stopping treatment. Your doctor will be happy to discuss any questions you may have with your treatment.
Tell all doctors, dentists, and pharmacists who are treating you that you are taking RISPERDAL.
Tell your doctor if you are not pregnant or planning to become pregnant.
If you are pre-menopausal, tell your doctor if you do not have a period for more than six months while using RISPERDAL, even if you are not pregnant.
Tell your doctor immediately if you notice any involuntary movements of the tongue, mouth, cheeks or jaw which may progress to the arms and legs. These may be symptoms of a condition called Tardive Dyskinesia, which can develop in people taking antipsychotic medicines, including RISPERDAL. This condition is more likely to occur during longer term treatment and in older women. In very rare cases, these symptoms may be permanent. However, if detected early, these symptoms are usually reversible.
Be careful during strenuous exercise or exposure to extreme heat. Try to drink plenty of water.
Do not drink alcohol. RISPERDAL can increase the effects of alcohol.
Things to be careful of
Ask your doctor or pharmacist before taking any other medicines.
RISPERDAL can increase the effects of medicines which slow your reactions. Always ask your doctor or pharmacist before taking other medicines, including herbal treatments and medicines that can be bought in a pharmacy or supermarket.
Avoid driving or operating machinery until you are sure RISPERDAL does not affect your alertness. RISPERDAL may cause dizziness or light-headedness in some people, especially after the first dose. Make sure you know how you react to RISPERDAL before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy.
If the medicine makes you feel light-headed, dizzy or faint, be careful when getting up from a sitting or lying position. Getting up slowly may help.
Avoid excessive eating. There is a possibility of weight gain when taking RISPERDAL. Your doctor may monitor your body weight or recommend strategies to assist with weight management.
Side Effects
All medicines can have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor has weighed the risks of using this medicine against the benefits they expect it will have for you.
All medicines can have side effects. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking RISPERDAL
Tell your doctor if you notice any of the following and they worry you:
- difficulty thinking, working or carrying out your usual daily activities because of:
- headache
- trembling, muscle weakness, unsteadiness on your feet, lack of coordination or slow, shuffling walk (symptoms of Parkinsonism).
- lack of energy, drowsiness or excessive sleeping during the day, sleeplessness or difficulty in concentrating
- difficulty speaking
- blurred vision
- fainting
- dizziness
- any problems with confusion or unsteadiness
- pains in parts of your body, e.g. in the neck, back, ear, hands or feet
- falling - muscle, joint, nerve or movement changes such as:
- shaking or trembling
- fatigue or weakness
- muscle stiffness
- restlessness in the legs or difficulty sitting still
- uncontrolled muscle spasms, twitching, jerky or writhing movements
- muscle aches or pain
- joint swelling or pain
- walking abnormally or with difficulty
- abnormal posture, such as rigid body movements and persistent abnormal positions of the body - behavioural changes such as:
- irritability or agitation
- unusual anxiety or nervousness - other changes such as:
- cold or "flu-like" symptoms e.g. cough, blocked or runny nose, sneezing, sore throat
- feeling of tension or fullness in the nose, cheeks and behind your eyes, sometimes with a throbbing ache, fever, stuffy nose and loss of the sense of smell (signs of sinusitis)
- tiredness, headaches, being short of breath when exercising, dizziness and looking pale (signs of decreased red blood cells)
- fever, chills, shortness of breath, cough, phlegm and occasionally blood (signs of pneumonia)
- nosebleeds
- discharge with itching of the eyes and crusty eyelids
- unexplained weight gain
- unexplained increase or decrease in appetite
- indigestion, stomach discomfort or pain, diarrhoea or constipation
- nausea or vomiting
- dry mouth or excessive thirst
- drooling
- difficulty swallowing
- acne
- dry skin
- rash, red skin or itchy skin
- thickening of the skin resulting in warts, corns, calluses
- skin infection
- swelling of any part of your body, e.g. hands, ankles or feet
- inability to or feeling burning pain when passing urine
- some loss of bladder control
- bedwetting
- frequent daytime urination in children
- sexual function disturbances - problems with ejaculation
- breast abnormalities - breast discomfort or swelling or unusual secretion of breast milk
- missed or irregular menstrual periods
- dizziness on standing up, especially when getting up from a sitting or lying down position
- shortness of breath
- chest pain or discomfort
- an increase of CPK (creatine phosphokinase) in your blood, an enzyme which is sometimes released with muscle breakdown.
These can only be detected by blood tests that your doctor may ask to be done.
These are mild side effects of RISPERDAL but may require medical attention.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- Signs of heart or blood pressure problems including:
- fainting, blurry vision, light-headedness or dizziness particularly on standing that persists despite sitting or lying down again
- very fast heart rate, slowed heart rate, heart rhythm irregularities - Signs of lung problems including:
- sudden shortness of breath, trouble breathing, wheezing or gasping when you breathe, light-headedness or dizziness - signs of high blood sugar or diabetes such as:
- unusual thirst, tiredness, upset stomach or need to urinate more often than usual - body temperature changes such as:
- fever
- unexplained high body temperature, excessive sweating or rapid breathing
- severe muscle stiffness or fits - involuntary movements of the tongue, face, mouth, jaw, arms, legs or trunk
- severe or life-threatening rash with blisters and peeling skin that may start in and around the mouth, nose, eyes, and genitals and spread to other areas of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis)
- rash, itching or hives on the skin; shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body.
If you have them, you may have had a serious allergic reaction to RISPERDAL. - sudden weakness or numbness of the face, arms, or legs, especially on one side, or instances of slurred speech (these are called mini-strokes)
These are very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some patients.
Do not hesitate to report any other side effects to your doctor or pharmacist.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using RISPERDAL
Storage
Keep RISPERDAL tablets in a dry place where the temperature stays below 25°C. Protect from light.
Keep RISPERDAL oral solution below 30°C. The oral solution should not be refrigerated.
Do not store it or any medicines in the bathroom or near a sink. Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not use RISPERDAL beyond the date (month and year) printed on the pack after the letters "EXP", even if it has been stored properly. Medicines cannot be stored indefinitely.
Do not use RISPERDAL if the appearance of the tablets or oral solution has changed.
Disposal
If your doctor tells you to stop taking RISPERDAL or if it has passed the expiry date, ask your pharmacist what to do with any that are left over.
Product Description
What it looks like
RISPERDAL Tablets
You can identify RISPERDAL Tablets by their colour and shape. This is important because there are 5 types of RISPERDAL, each containing a different amount of risperidone:
- 0.5 mg brownish red, oblong biconvex grooved tablets marked with "Ris 0.5" on the grooved side and "JANSSEN" on the other side.
- 1 mg white, oblong grooved tablets marked "Ris 1" on the grooved side.
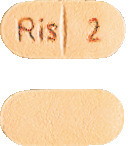
- 2 mg orange, oblong grooved tablets marked "Ris 2" on the grooved side.
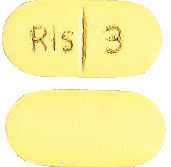
- 3 mg yellow, oblong grooved tablets marked "Ris 3" on the grooved side.
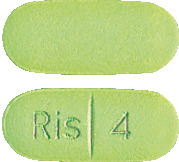
- 4 mg green, oblong grooved tablets marked "Ris 4" on the grooved side.
All strengths come in boxes of 60 tablets, except the 0.5 mg which comes in a box of 20 tablets.
RISPERDAL oral solution
RISPERDAL oral solution is a clear, colourless solution. It is packed in an amber bottle (30 or 100mL) with a pipette which measures the dose in milligrams (mg) and millilitres (mL). One full pipette contains 3 mL (or 3 mg) of oral solution. The smallest amount you can measure with the pipette is 0.25 mL.
Ingredients
The active ingredient in RISPERDAL is risperidone.
RISPERDAL Tablets
RISPERDAL tablets contain either 0.5, 1, 2, 3 or 4 mg (milligrams) of risperidone. The tablets also contain lactose monohydrate, maize starch, microcrystalline cellulose, hypromellose, magnesium stearate, colloidal anhydrous silica, sodium lauryl sulphate, propylene glycol.
The 0.5 mg tablets also contain titanium dioxide, purified talc and red iron oxide.
The 2 mg tablets also contain titanium dioxide, purified talc and sunset yellow FCF.
The 3 mg tablets also contain titanium dioxide, purified talc and quinoline yellow.
The 4 mg tablets also contain titanium dioxide, purified talc, quinoline yellow and indigo carmine.
RISPERDAL oral solution
The RISPERDAL oral solution contains 1 mg/mL of risperidone, as well as tartaric acid, benzoic acid, sodium hydroxide and purified water.
Sponsor
Janssen-Cilag Pty Ltd
1-5 Khartoum Road Macquarie Park NSW 2113
Telephone: 1800 226 334
NZ Office: Auckland, New Zealand
Telephone: 0800 800 806
Registration Numbers
0.5mg Tablet - AUST R 71849
1 mg Tablet - AUST R 47008
2 mg Tablet - AUST R 48486
3 mg Tablet - AUST R 47010
4 mg Tablet - AUST R 47011
1 mg/mL Oral Solution - AUST R 56444
This leaflet was prepared in August 2021.
Published by MIMS October 2021



 For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided (see Table 2).
For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided (see Table 2). Patients experiencing somnolence may benefit from a switch in dosing from once daily to either once daily at bedtime or twice daily.
Patients experiencing somnolence may benefit from a switch in dosing from once daily to either once daily at bedtime or twice daily.


 ADRs reported with risperidone and/or paliperidone by < 1% of Risperdal treated subjects in a pooled dataset of 23 double blind, placebo controlled pivotal studies (9 in adults, 6 in elderly patients with dementia, and 8 in paediatric patients) are shown in Table 7.
ADRs reported with risperidone and/or paliperidone by < 1% of Risperdal treated subjects in a pooled dataset of 23 double blind, placebo controlled pivotal studies (9 in adults, 6 in elderly patients with dementia, and 8 in paediatric patients) are shown in Table 7. ADRs reported with risperidone and/or paliperidone in other clinical trials but not reported by Risperdal treated subjects in a pooled dataset of 23 double blind, placebo controlled pivotal studies are shown in Table 8.
ADRs reported with risperidone and/or paliperidone in other clinical trials but not reported by Risperdal treated subjects in a pooled dataset of 23 double blind, placebo controlled pivotal studies are shown in Table 8.
 There have also been reports of benign pituitary adenoma that were temporally related, but not necessarily causally related, to risperidone therapy.
There have also been reports of benign pituitary adenoma that were temporally related, but not necessarily causally related, to risperidone therapy. The rate of discontinuation from the pooled studies was similar for patients receiving placebo (30.2%), risperidone (33.5%) and haloperidol (29.6%). In the combined analysis, risperidone, at a daily dose above 0.75 mg, effectively reduces the severity (measured by means of the Behave-AD) and frequency (measured by the CMAI) of aggressiveness symptoms in this patient population. Reductions in Behave-AD aggressiveness scores and on each of the aggressive clusters of the CMAI were significantly greater with risperidone (doses above 0.75 mg/day) than placebo at endpoint in both studies and in the combined analysis. Reductions in CMAI total aggressive scores declined throughout the studies in the risperidone patients but changed minimally after week 2 in patients receiving haloperidol or placebo.
The rate of discontinuation from the pooled studies was similar for patients receiving placebo (30.2%), risperidone (33.5%) and haloperidol (29.6%). In the combined analysis, risperidone, at a daily dose above 0.75 mg, effectively reduces the severity (measured by means of the Behave-AD) and frequency (measured by the CMAI) of aggressiveness symptoms in this patient population. Reductions in Behave-AD aggressiveness scores and on each of the aggressive clusters of the CMAI were significantly greater with risperidone (doses above 0.75 mg/day) than placebo at endpoint in both studies and in the combined analysis. Reductions in CMAI total aggressive scores declined throughout the studies in the risperidone patients but changed minimally after week 2 in patients receiving haloperidol or placebo. Following completion of study 1, 63 patients entered an open label extension for up to 4 additional months. Thirty nine patients who were clinically stable and who showed a positive response to risperidone after 6 months were then randomised to receive Risperdal or placebo during an 8 week, double blind withdrawal period. The relapse rate was 11/16 and 2/16 in placebo and Risperdal treated patients respectively (odds ratio 15.4, 95% confidence limits 2.50, 95.05).
Following completion of study 1, 63 patients entered an open label extension for up to 4 additional months. Thirty nine patients who were clinically stable and who showed a positive response to risperidone after 6 months were then randomised to receive Risperdal or placebo during an 8 week, double blind withdrawal period. The relapse rate was 11/16 and 2/16 in placebo and Risperdal treated patients respectively (odds ratio 15.4, 95% confidence limits 2.50, 95.05). As few autistic children with an IQ > 84 are seen, there is limited clinical experience with Risperdal in such children. Experience in autistic adolescents is also limited.
As few autistic children with an IQ > 84 are seen, there is limited clinical experience with Risperdal in such children. Experience in autistic adolescents is also limited. C23H27FN4O2.
C23H27FN4O2.