What is in this leaflet
This leaflet answers some common questions about ROSUZET COMPOSITE PACK. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking ROSUZET COMPOSITE PACK against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What ROSUZET COMPOSITE PACK is used for
ROSUZET COMPOSITE PACK helps to lower cholesterol levels. It is used in people whose cholesterol levels are too high and when diet alone cannot lower these levels adequately.
What is high cholesterol
Cholesterol is one of several fatty substances found in the bloodstream. Your total cholesterol is made up mainly of LDL and HDL cholesterol.
LDL cholesterol is often called “bad” cholesterol because it can build up in the walls of your arteries forming plaque. Eventually this plaque build-up can lead to a narrowing of the arteries. This narrowing can slow or block blood flow to vital organs such as the heart and brain. This blocking of blood flow can result in a heart attack or stroke.
HDL cholesterol is often called “good” cholesterol because it helps keep the bad cholesterol from building up in the arteries and protects against heart disease.
How ROSUZET COMPOSITE PACK works
ROSUZET COMPOSITE PACK is a combination pack, which contains two different medicines. One is Ezetrol (ezetimibe) and the other is rosuvastatin (rosuvastatin calcium). ROSUZET COMPOSITE PACK reduces elevated total-cholesterol, LDL ('bad') cholesterol and increases HDL ('good') cholesterol.
ROSUZET COMPOSITE PACK works by decreasing the absorption of cholesterol in the small intestine and by reducing the amount of cholesterol made in the liver.
Your doctor may have prescribed ROSUZET COMPOSITE PACK for another reason.
Ask your doctor if you have any questions about why ROSUZET COMPOSITE PACK has been prescribed for you.
ROSUZET COMPOSITE PACK is not addictive.
ROSUZET COMPOSITE PACK is available only with a doctor's prescription.
Use in children
ROSUZET COMPOSITE PACK is not recommended for use in children.
Before you take ROSUZET COMPOSITE PACK
When you must not take it
Do not take ROSUZET COMPOSITE PACK if:
- you have an allergy to ROSUZET COMPOSITE PACK or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin - you have had muscle pain, tenderness or weakness from other medicines used to treat high cholesterol or triglycerides.
- you are pregnant or intend to become pregnant.
If you are a woman of child-bearing age and are taking ROSUZET COMPOSITE PACK, use a proven method of birth control to avoid pregnancy.
The medicine may affect your developing baby if you take it during pregnancy. - you are breast-feeding.
This medicine may pass into breast milk and affect your baby. - you have active liver disease.
- you are taking any medicine containing fusidic acid (for bacterial infections)
- the expiry date on the pack has passed or if the packaging is torn or shows signs of tampering.
If you take ROSUZET COMPOSITE PACK after the expiry date has passed, it may not work.
Return any expired or damaged ROSUZET COMPOSITE PACK to your pharmacist for disposal
Do not take ROSUZET COMPOSITE PACK together with fenofibrate if:
- you have gall bladder disease.
Do not use ROSUZET COMPOSITE PACK 10mg+40mg if you:
- have low thyroid hormone levels (hypothyroidism)
- have a personal or family history of hereditary muscular disorders
- have a previous history of muscular problems from using other medicines used to treat high cholesterol or triglycerides
- have a history of very heavy alcohol consumption
- have severe kidney impairment
- are of Asian descent
- are taking another type of lipid-lowering medicine called a fibrate
Before taking ROSUZET COMPOSITE PACK 10mg+40mg, talk to your doctor about situations that can increase rosuvastatin blood levels.
Also talk to your doctor if you are not sure whether you should start taking ROSUZET COMPOSITE PACK.
Before you start to take it
Your doctor will ask you to have your liver function tested before you start to take ROSUZET COMPOSITE PACK.
Tell your doctor if:
- you have any allergies to any other medicines or any other substances, such as foods, preservatives or dyes.
you have, or have had, any medical condition, including:
- liver problems
Your doctor will do a blood test to make sure you have no problems with your liver. - kidney problems
- personal or family history of muscle problems
- muscle pain, tenderness or weakness from other medicines used to treat high cholesterol or triglycerides
- unexplained muscle aches or pains in your muscle
- breathing problems
- low thyroid hormone levels (hypothyroidism)
- diabetes or risk factors for diabetes
- you drink alcohol regularly.
Excessive alcohol consumption may not be safe in patients taking ROSUZET COMPOSITE PACK. - you have or have had myasthenia gravis (a disease causing general muscle weakness including in some cases muscles used for breathing) or ocular myasthenia (a disease causing eye muscle weakness) as statins may lead to occurrence of myasthenia or aggravate the condition.
If you have not told your doctor about any of the above, tell him/her before you start taking ROSUZET COMPOSITE PACK.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines should not be taken with ROSUZET COMPOSITE PACK, including:
- fusidic acid, antibiotic used to treat infection
- fenofibrate (a medicine to lower triglyceride levels) if you have gall bladder disease
Some medicines may be affected by ROSUZET COMPOSITE PACK, or may affect how well it works, or may increase the risk of side effects with ROSUZET COMPOSITE PACK. You may need different amounts of your medicines, or you may need to take different medicines or take your medicines at different times. Your doctor will advise you.
Tell your doctor if...
Tell your doctor if you are taking any of the following:
- bile acid sequestrants, such as colestyramine, used to lower cholesterol levels
- other medicines to lower cholesterol or triglyceride levels, for example, gemfibrozil, other fibrates, Vitamin B3
- warfarin or fluindione or ticagrelor used to prevent blood clots
- HIV protease inhibitors, used to treat HIV infection
- hepatitis C antiviral agents, such as elbasvir or grazoprevir, used to treat the hepatitis C virus infection
- colchicine, used to treat gout
- ciclosporin, used to suppress the immune system
- antacids, used to treat reflux or ulcers. ROSUZET COMPOSITE PACK should be taken 2 hours before or 2 hours after taking antacid.
- daptomycin, a medicine used to treat complicated skin and skin structure infections and bacteraemia
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking ROSUZET COMPOSITE PACK.
If you have not told your doctor about any of the above, tell them before you start taking ROSUZET COMPOSITE PACK.
How to take ROSUZET COMPOSITE PACK
Take ROSUZET COMPOSITE PACK only when prescribed by your doctor.
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand these instructions, ask your doctor or pharmacist for help.
If you are currently taking a medicine that contains ezetimibe and/or rosuvastatin which are contained in ROSUZET COMPOSITE PACK:
- stop taking your current medicine(s) that contain ezetimibe and/or rosuvastatin as this may result in you taking more medicine than you need
- take the remaining medicine(s) to your pharmacist for safe disposal
Check with your doctor if you are not sure about the medicines you are taking.
How much to take
There are two different medicines in ROSUZET COMPOSITE PACK. Your doctor will prescribe a strength that is appropriate for you. It is very important that you take ROSUZET COMPOSITE PACK exactly as follows:
Take one Ezetrol (ezetimibe) tablet and one OGN Rosuvastatin (rosuvastatin) tablet at the same time once a day.
If your doctor has increased the strength of ROSUZET COMPOSITE PACK tablets you take, do not take multiple doses of any remaining supplies of lower strength ROSUZET COMPOSITE PACK tablets instead of the new higher strength tablets as this will result in you taking more medicine (ezetimibe) than you need.
How to take it
Swallow the tablets whole with a full glass of water.
Do not chew or crush the tablets.
When to take it
ROSUZET COMPOSITE PACK can be taken at any time of the day. However, ROSUZET COMPOSITE PACK should be taken at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take ROSUZET COMPOSITE PACK with or without food.
Taking ROSUZET COMPOSITE PACK with other cholesterol-lowering agents
Your doctor may ask you to take ROSUZET COMPOSITE PACK with other cholesterol-lowering agents such as bile acid sequestrants.
If you are taking a bile acid sequestrant, such as colestyramine, take ROSUZET COMPOSITE PACK either at least two hours before or four hours after taking the bile acid sequestrant.
How long to take it
Take ROSUZET COMPOSITE PACK every day and continue taking it for as long as your doctor tells you.
ROSUZET COMPOSITE PACK helps to lower your cholesterol levels but does not cure your condition. If you stop taking ROSUZET COMPOSITE PACK your cholesterol levels may rise again.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting a side effect.
If you are not sure what to do, talk to your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much ROSUZET COMPOSITE PACK. Do this even if there are no signs of discomfort or poisoning. You or anyone else may need urgent medical attention.
While you are taking ROSUZET COMPOSITE PACK
Things you must do
If you become pregnant while you are taking ROSUZET COMPOSITE PACK, stop taking it and tell your doctor immediately.
Keep all your doctor's appointments.
Even if you are taking medicines to treat high cholesterol, it is important to have your cholesterol measured regularly. You should also know your cholesterol levels and goals.
Your doctor will ask you to have your liver function tested from time to time while you are taking ROSUZET COMPOSITE PACK. This may help prevent unwanted side effects.
Your doctor may adjust your dose depending on the result of the test.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking ROSUZET COMPOSITE PACK.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking ROSUZET COMPOSITE PACK.
Things you must not do
Do not take ROSUZET COMPOSITE PACK to treat any other conditions unless your doctor tells you to.
Do not give ROSUZET COMPOSITE PACK to anyone else, even if they have the same condition as you.
Things to be careful of
Avoid drinking large quantities of alcohol. Drinking large quantities of alcohol while taking ROSUZET COMPOSITE PACK may increase your chance of getting liver problems.
Be careful driving or operating machinery until you know how ROSUZET COMPOSITE PACK affects you. There have been side effects reported with ROSUZET COMPOSITE PACK that may affect your ability to drive or operate machinery. Individual responses to ROSUZET COMPOSITE PACK may vary.
Things that may help your condition
Lifestyle Changes -
This includes a cholesterol-lowering diet, increasing physical activity, and weight management. Ask your doctor for advice before increasing physical activity.
Medicines -
Cholesterol-lowering medicines are used together with lifestyle changes to help lower cholesterol.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ROSUZET COMPOSITE PACK.
ROSUZET COMPOSITE PACK helps most people with high cholesterol levels, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if...
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- diarrhoea
- muscle aches, spasms, tiredness or weakness
- constipation
- nausea
- stomach or belly pain
- heartburn, indigestion or wind
- aches and pain
- pain in arms and legs
- trouble sleeping
- dizziness
- headache
- tingling or numbness of the hands or feet
- hot flush
- shortness of breath
- unusual tiredness or weakness
- generally feeling unwell
- memory loss
- itchy skin
- decreased appetite
- dry mouth
- swelling, especially in the hands and feet
- cough
Tell your doctor if you have been diagnosed with:
- inflammation of the pancreas
- gallstones
- inflammation of the gallbladder
- elevation in some laboratory blood tests of liver or muscle function
- depression
- elevation in blood glucose levels
- diabetes
- high blood pressure
- thyroid function abnormalities
The above list includes the more common and uncommon side effects of your medicine. They are usually mild and short-lived.
Tell your doctor if you notice a significant increase in your need to urinate or if you are significantly more hungry or thirsty than usual.
Tell your doctor immediately if...
Tell your doctor immediately if you notice any of the following:
- skin rash and hives
- raised red rash, sometimes with circular-shaped lesions
- steady abdominal pain with nausea and vomiting
- joint pain
- bleeding or bruising more easily than normal
- yellowing of the skin and eyes
- dark coloured urine
- light coloured bowel motions
- problems with breathing including shortness of breath, persistent cough and fever
- weakness in your arms or legs that worsens after periods of activity, double vision or drooping of your eyelids, difficulty swallowing, or shortness of breath (symptoms of myasthenia).
These may be serious side effects. You may need urgent medical attention.
Go to hospital if...
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, mouth, throat or tongue which may cause difficulty in swallowing or breathing.
These may be serious side effects. If you have them, you may have had a serious allergic reaction to ROSUZET COMPOSITE PACK. You may need urgent medical attention or hospitalisation. These side effects are very rare. - chest pain
- unexpected muscle pain, tenderness, or weakness not caused by exercise, particularly if you also have a fever or generally feel unwell.
This may be a serious side effect. This is because on rare occasions, muscle problems can be serious including muscle breakdown resulting in kidney damage. You may need urgent medical attention. - severe skin problems.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After taking ROSUZET COMPOSITE PACK
Storage
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store ROSUZET COMPOSITE PACK or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking ROSUZET COMPOSITE PACK or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
ROSUZET COMPOSITE PACK comes in four different strengths:
- ROSUZET COMPOSITE PACK 10 mg + 5 mg
- ROSUZET COMPOSITE PACK 10 mg + 10 mg
- ROSUZET COMPOSITE PACK 10 mg + 20 mg
- ROSUZET COMPOSITE PACK 10 mg + 40 mg
Ezetrol 10 mg tablet is a white to off-white, capsule-shaped tablet with "414" marked on one side.
OGN Rosuvastatin 5 mg is a brown, round, film coated tablet.
OGN Rosuvastatin 10 mg is a brown, round, film coated tablet with “RSV 10” marked on one side.
OGN Rosuvastatin 20 mg is a brown, round, film coated tablet with “RSV 20” marked on one side.
OGN Rosuvastatin 40 mg is a brown, round, film coated tablet with “RSV 40” marked on one side.
Ezetrol 10mg and OGN Rosuvastatin (5, 10, 20, 40 mg) are supplied in 10-day calendar packs of 10 Ezetrol and 10 OGN Rosuvastatin tablets.
A trade pack contains 3 x 10-day calendar packs.
A starter pack contains 1 x 10-day calendar pack.
Ingredients
Ezetrol
Active ingredient:
- Ezetimibe 10 mg per tablet.
Inactive ingredients:
- Croscarmellose sodium
- Magnesium stearate
- Lactose monohydrate
- Microcrystalline cellulose
- Povidone
- Sodium lauryl sulfate
OGN Rosuvastatin
Active ingredient:
- Rosuvastatin (as rosuvastatin calcium) 5 mg per tablet.
- Rosuvastatin (as rosuvastatin calcium) 10 mg per tablet.
- Rosuvastatin (as rosuvastatin calcium) 20 mg per tablet.
- Rosuvastatin (as rosuvastatin calcium) 40 mg per tablet.
Inactive ingredients:
- Purified talc
- Microcrystalline cellulose
- Silicon dioxide
- Colloidal anhydrous silica
- Maize starch
- Sodium stearylfumarate
- Hypromellose
- Mannitol
- Lactose
- Macrogol 6000
- Titanium dioxide
- Iron oxide yellow
- Iron oxide red
Date of Preparation
This Consumer Medicine Information was prepared in October 2023.
Ask your pharmacist about any updates.
Supplier
ROSUZET COMPOSITE PACK is supplied in Australia by:
Organon Pharma Pty Limited
Building A 26 Talavera Road
Macquarie Park NSW 2113
Australia
Australian Registration Numbers
ROSUZET COMPOSITE PACK 10 mg + 5 mg - Aust R 203690
ROSUZET COMPOSITE PACK 10 mg + 10 mg - Aust R 203694
ROSUZET COMPOSITE PACK 10 mg + 20 mg - Aust R 203692
ROSUZET COMPOSITE PACK 10 mg + 40 mg - Aust R 203687
S-CCPPI-OG0653H-T-062023
Published by MIMS December 2023

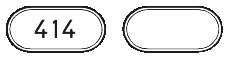


 In this study, the incidence of clinically important elevations in serum transaminases (ALT ≥ 3 x ULN, consecutive) was 0.5% (n = 1) for patients treated with ezetimibe + rosuvastatin and 0% for patients in the rosuvastatin only treatment group. No patients in either group had clinically significant elevations in AST. Clinically important elevations in creatine kinase (CK ≥ 10 x ULN) were seen in 0.5% (n = 1) of patients in the rosuvastatin only treatment group and not seen in patients treated with ezetimibe + rosuvastatin.
In this study, the incidence of clinically important elevations in serum transaminases (ALT ≥ 3 x ULN, consecutive) was 0.5% (n = 1) for patients treated with ezetimibe + rosuvastatin and 0% for patients in the rosuvastatin only treatment group. No patients in either group had clinically significant elevations in AST. Clinically important elevations in creatine kinase (CK ≥ 10 x ULN) were seen in 0.5% (n = 1) of patients in the rosuvastatin only treatment group and not seen in patients treated with ezetimibe + rosuvastatin. In addition, the following common or uncommon drug-related adverse experiences were reported in clinical trials in patients taking ezetimibe co-administered with a statin and at a greater incidence than statin administered alone, or in patients taking ezetimibe alone and at a greater incidence than placebo.
In addition, the following common or uncommon drug-related adverse experiences were reported in clinical trials in patients taking ezetimibe co-administered with a statin and at a greater incidence than statin administered alone, or in patients taking ezetimibe alone and at a greater incidence than placebo.
 The secondary endpoints were percent change from baseline in other lipid and lipoprotein parameters and percent change from baseline in LDL-C at Week 6. Ezetimibe 10 mg added to on-going rosuvastatin therapy (5 or 10 mg) significantly lowered total-cholesterol, non-HDL-C and Apo-B, compared with doubling of the rosuvastatin dose (p < 0.001) and resulted in a significantly greater proportion of patients reaching their LDL-C goal compared with doubling the baseline dose of rosuvastatin (10 mg or 20 mg) (59.4% vs. 30.9%, adjusted odds ratio = 4.5 with a 95% CI of (2.9, 6.9); p < 0.001). LDL-C treatment goals were < 1.8 mmol/L for patients at high risk for CHD with AVD and < 2.6 mmol/L for patients at moderately high risk and high risk for CHD without AVD.
The secondary endpoints were percent change from baseline in other lipid and lipoprotein parameters and percent change from baseline in LDL-C at Week 6. Ezetimibe 10 mg added to on-going rosuvastatin therapy (5 or 10 mg) significantly lowered total-cholesterol, non-HDL-C and Apo-B, compared with doubling of the rosuvastatin dose (p < 0.001) and resulted in a significantly greater proportion of patients reaching their LDL-C goal compared with doubling the baseline dose of rosuvastatin (10 mg or 20 mg) (59.4% vs. 30.9%, adjusted odds ratio = 4.5 with a 95% CI of (2.9, 6.9); p < 0.001). LDL-C treatment goals were < 1.8 mmol/L for patients at high risk for CHD with AVD and < 2.6 mmol/L for patients at moderately high risk and high risk for CHD without AVD.

