SUMMARY CMI
SABRIL®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Sabril?
Sabril contains the active ingredient vigabatrin. Sabril is used in addition to other medicines to treat epilepsy.
For more information, see Section 1. Why am I using Sabril? in the full CMI.
2. What should I know before I take Sabril?
Do not take if you have ever had an allergic reaction to Sabril or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take Sabril? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Sabril and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take Sabril?
- Sabril tablets and sachets are taken by mouth either once or twice a day.
- Take Sabril at about the same time each day.
More instructions can be found in Section 4. How do I take Sabril? in the full CMI.
5. What should I know while using Sabril?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Sabril? in the full CMI.
6. Are there any side effects?
The less serious side effects are drowsiness, nervousness, irritability, depression, dizziness, confusion, headache, tiredness difficulty sleeping, joints pain, impaired concentration, weight gain, difficulty in speaking or slurred speech, lack of coordination agitation, excitation, aggression, tremors, nausea, vomiting, abdominal pain and unusual hair loss or thinning. Serious side effects are an increase in the number of seizures, changes in your behaviour or mood, severe tiredness or confusion, changes in your eyesight and suicidal thoughts/attempts.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
SABRIL®
Active ingredient(s): vigabatrin
Consumer Medicine Information (CMI)
This leaflet provides important information about using Sabril. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Sabril.
Where to find information in this leaflet:
1. Why am I using Sabril?
2. What should I know before I take Sabril?
3. What if I am taking other medicines?
4. How do I take Sabril?
5. What should I know while using Sabril?
6. Are there any side effects?
7. Product details
1. Why am I using Sabril?
Sabril contains the active ingredient vigabatrin.
Sabril is a medicine used to help control epilepsy, a condition where you have repeated fits or convulsions.
Sabril is an anticonvulsant. It works by stopping the breakdown of an important chemical transmitter ("messenger") in the brain and this helps reduce seizure activity.
Sabril is used in addition to other medicines to treat epilepsy.
Your doctor, however, may have prescribed Sabril for another reason.
Ask your doctor if you have any questions about why it has been prescribed for you.
2. What should I know before I take Sabril?
Warnings
Do not take Sabril if:
- you are allergic to vigabatrin, or any of the ingredients listed at the end of this leaflet.
- you are pregnant or intend to become pregnant unless your doctor tells you to. It may affect your developing baby if you take it during pregnancy.
- you are breast-feeding or planning to breast-feed unless your doctor tells you to. Sabril passes into breast milk and there is a possibility your baby may be affected.
- Always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
- take any medicines for any other condition
- plan to have surgery
- have any other medical conditions, especially the following:
- kidney problems
- nervous or mental illness
- depression
- psychosis, a severe mental condition in which the person is unable to think and judge clearly. - have allergies to:
- any of the ingredients listed at the end of this leaflet
- any other medicines
- any other substances, such as foods, preservatives, or dyes
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Tell your doctor if you are pregnant or intend to become pregnant.
Like most medicines for epilepsy, Sabril may affect your unborn baby. However, it is very important to control your epilepsy while you are pregnant since seizures may also affect your baby. Your doctor will help you decide whether you should take Sabril during pregnancy
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Sabril passes into breast milk. Your doctor will discuss the risks and benefits of using it if you are breast-feeding or planning to breast-feed.
Eyesight
Your doctor may want to test your eyesight before you start taking Sabril and during the course of your treatment so that any changes in your sight can be detected.
Some anti-epilepsy medicines may affect eyesight. In rare cases, patients treated with Sabril have experienced changes to their peripheral vision (i.e., they had trouble seeing things that were not directly in front of them).
Please ask your doctor if you want more information about this.
- If you have not told your doctor about any of the above, tell them before you take Sabril.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with the absorption of Sabril.
- These include phenytoin, a medicine used to treat seizures.
Other medicines can increase the drowsiness that Sabril causes.
- These include clonazepam, a medicine used as a sedative or to treat anxiety.
These medicines may be affected by Sabril. You may need to use different amounts of your medicine or take different medicines.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Sabril.
4. How do I take Sabril?
How much to take
- Sabril tablets and sachets are taken by mouth either once or twice a day.
- The dose of Sabril varies between patients. Your doctor may have prescribed a different dose.
Follow all directions given to you by your doctor and pharmacist carefully.
These directions may differ from the information contained in this leaflet.
Ask your doctor or pharmacist if you are unsure of the correct dose for you.
If you take the wrong dose, Sabril may not work as well and your problem may not improve.
When to take Sabril
- Take Sabril at about the same time each day.
- Taking Sabril at the same time each day will have the best effect. It will also help you remember when to take it.
- You can take Sabril with or without food.
If you are not sure when to take it, ask your doctor or pharmacist.
How to take Sabril
Tablets
- Swallow your tablet(s) whole with plenty of water. Do not crush or chew the tablet(s).
Sachets
- The contents of the sachets should be dissolved in half a glass of water, juice or soft drink just before you plan to take it.
- Once dissolved, the solution should be taken immediately. It will dissolve easily and has no taste or smell.
- Once mixed, any remaining solution should be discarded within 24 hours.
How long to take Sabril
- Do not stop taking Sabril until your doctor tells you to.
- Ask your doctor or pharmacist if you are not sure how long to take the medicine for.
If you forget to take Sabril
Do not take a double dose to make up for the dose you missed.
- If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
- If there is still a long time to go before your next dose, take it as soon as you remember, and then go back to taking your dose as you would normally.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for hints.
If you take too much Sabril
If you think that you have taken too much Sabril, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre for advice
- Australia 13 11 26 or
- New Zealand 0800 POISON or 0800 764766), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Sabril?
Things you should do
- Tell all the doctors, dentists and pharmacists that are treating you that you are taking Sabril.
- If you are about to start taking any new medicine, tell your doctor and pharmacist that you are taking Sabril.
- If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
- If you become pregnant while you are taking Sabril, tell your doctor immediately.
- Tell your Doctor immediately or go to the Accident and Emergency department of your nearest hospital if you have any thoughts of harming yourself or committing suicide.
Things you should not do
- Do not stop taking Sabril until your doctor tells you to.
- Do not give this medicine to anyone else, even if they have the same condition as you.
- Do not take more than the recommended dose unless your doctor or pharmacist tells you to.
- Do not use this medicine to treat any other complaints unless your doctor tells you to.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Sabril affects you.
Sabril may cause dizziness or light-headedness in some people. Make sure you know how you react to Sabril before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy or light-headed. This effect decreases with time.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep your tablets or sachets in the container until it is time to take them.
- Keep Sabril in a cool dry place where the temperature stays below 30°C.
- Do not store Sabril or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
- Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it.
Do not take it after the expiry date (EXP) printed on the pack.
If you take this medicine after the expiry date has passed, it may not work as well.
Do not take it if the packaging is torn or shows signs of tampering.
Getting rid of any unwanted medicine
If your doctor tells you to stop taking Sabril tablets or sachets or the expiry date has passed, ask your pharmacist what to do with any tablets or sachets that are left over.
Return any unused medicine to your pharmacist.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Head and neurology related
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Allergic reaction
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. Stop taking the Sabril if you have signs of an allergic reaction. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects as follows. Australia: Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. New Zealand: Medsafe online at www.medsafe.govt.nz/safety/report-a-problem.asp
By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Sabril contains
Sabril does not contain gluten, sucrose, tartrazine or any other azo dyes.
Tablets
| Active ingredient (main ingredient) | Each tablet contains: 500 mg of vigabatrin. |
| Other ingredients (inactive ingredients) | povidone microcrystalline cellulose sodium starch glycollate magnesium stearate hypromellose titanium dioxide macrogol 8000 Opadry White OY-S-7298 |
Sachets
| Active ingredient (main ingredient) | Each sachet contains: 500 mg of vigabatrin. |
| Other ingredients (inactive ingredients) | povidone |
Do not take this medicine if you are allergic to any of these ingredients.
What Sabril looks like
Tablets
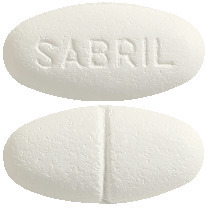
Sabril 500 mg are white to off-white, oval biconvex, film-coated tablets with a break-mark on one side and "SABRIL" or "SABRILEX" engraved on the other side. They come in a blister pack of 100 tablets (AUST R 150021).
Sachets
Sabril sachets contain 500 mg of vigabatrin. They come in a pack of 60 sachets (AUST R 52985).
Who distributes Sabril
Distributed in Australia by:
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park NSW 2113
Freecall: 1800 818 806
Email: [email protected]
Distributed in New Zealand by:
Pharmacy Retailing (NZ) Ltd t/a Healthcare Logistics
PO Box 62027
Sylvia Park Auckland 1644
Freecall: 0800 283 684
Email: [email protected]
This leaflet was prepared in August 2023.
sabril-ccdsv14-cmiv12-04aug23
Published by MIMS October 2023

 The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. Anyone considering prescribing vigabatrin or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behaviour. Should suicidal thoughts and behaviour emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behaviour and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behaviour, or the emergence of suicidal thoughts, behaviour, or thoughts about self harm. Behaviours of concern should be reported immediately to the treating doctor.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. Anyone considering prescribing vigabatrin or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behaviour. Should suicidal thoughts and behaviour emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behaviour and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behaviour, or the emergence of suicidal thoughts, behaviour, or thoughts about self harm. Behaviours of concern should be reported immediately to the treating doctor.
