SUMMARY CMI
STELAX Tablets
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Stelax?
Stelax contains the active ingredient baclofen. Stelax belongs to a group of medicines called muscle relaxants.
Stelax is used to reduce the stiffness or spasms in your muscles to help make you more mobile and able to manage your daily activities.
For more information, see Section 1. Why am I using Stelax? in the full CMI.
2. What should I know before I use Stelax?
Do not use if you have ever had an allergic reaction to Stelax or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Stelax? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Stelax and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Stelax?
- Your doctor will tell you how many tablets of Stelax to take.
- Do not exceed the recommended dose prescribed by your doctor.
- Treatment is usually started in hospital with small doses of Stelax. The dose is then gradually increased to an amount that works best for you.
More instructions can be found in Section 4. How do I use Stelax? in the full CMI.
5. What should I know while using Stelax?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Stelax? in the full CMI.
6. Are there any side effects?
Common side effects include daytime sleepiness, lack of energy, tiredness, dizziness or light-headiness, vertigo, confusion, headache, difficulty sleeping or nightmares, nausea, retching or vomiting, constipation, stomach cramps or diarrhoea, loss of appetite, stuffy nose, dry mouth, change in sense of taste, misuse, abuse and dependence, numbness, muscle weakness or spasms, swelling of ankles, blurred vision, ringing in the ears, frequent urination, excessive sweating, weight gain, impotence or inability to ejaculate, increased blood sugar, low body temperature.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
STELAX Tablets
Active ingredient(s): Baclofen
Consumer Medicine Information (CMI)
This leaflet provides important information about using Stelax. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Stelax.
Where to find information in this leaflet:
1. Why am I using Stelax?
2. What should I know before I use Stelax?
3. What if I am taking other medicines?
4. How do I use Stelax?
5. What should I know while using Stelax?
6. Are there any side effects?
7. Product details
1. Why am I using Stelax?
Stelax contains the active ingredient baclofen. Stelax belongs to a group of medicines called muscle relaxants.
Stelax is used to reduce the stiffness or spasms in your muscles to help make you more mobile and able to manage your daily activities. These spasms happen in various illnesses such as multiple sclerosis and diseases or injuries of the spinal cord.
2. What should I know before I use Stelax?
Warnings
Do not use Stelax if:
- you are allergic to baclofen, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
Check with your doctor if you:
- have any other medical conditions including mental illness, Parkinson's disease, seizures, stiffness and restriction of movement in a group of muscles, stomach ulcers, stroke or blood circulation problems, diabetes, a blood disorder known as porphyria, high blood pressure, difficulty urinating.
- have problems with your heart, kidney, liver or lungs.
- have a history of alcoholism, drink large amounts of alcohol, or have a history of drug abuse or dependence
- have had thoughts of self-harm or and suicidal thoughts
- take any medicines for any other condition
- have any questions about why this medicine has been prescribed to you.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Stelax and affect how it works. These include:
- any medicines that make you sleepy such as medicines used to help you sleep or calm you, pain relievers and medicines for cold or allergies
- medicines used to treat mood disorders, including tricyclic antidepressants, lithium and monoamine oxidase inhibitors (MAOIs)
- medicines used to treat diabetes
- medicines for high blood pressure
- medicines used to treat Parkinson's disease, including selegiline and levodopa and carbidopa.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Stelax.
4. How do I use Stelax?
How much to take
- Your doctor will tell you how many tablets of Stelax to take.
- Do not exceed the recommended dose prescribed by your doctor.
- Treatment is usually started in hospital with small doses of Stelax. The dose is then gradually increased to an amount that works best for you.
- If you are under the age of 16 or over 65, or you have kidney disease, your doctor may start you on a lower dose and increase slowly to prevent unwanted side effects.
- Follow the instructions provided and use Stelax until your doctor tells you to stop.
When to take Stelax
- Stelax is usually taken in 3 divided doses throughout the day.
- Your doctor may tell you to take it more or less often, depending on your situation.
How to take Stelax
- Swallow the tablets whole during meals with a glass of water or other liquid.
If you forget to take Stelax
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember and then go back to taking it as you would normally.
Do not take a double dose to make up for the dose you missed.
If you use too much Stelax
If you think that you have used too much Stelax, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
Symptoms of overdose include:
- Main symptoms such as drowsiness, breathing difficulties and being unconscious (coma).
- Other symptoms such as feeling confused, hallucinations, agitation, convulsions, blurred vision, unusual low muscle tone, sudden and involuntary muscle spasm, poor or absent reflexes, high or low blood pressure, slow, fast or irregular heartbeat, low body temperature, nausea, vomiting, diarrhoea, excessive salivation, trouble breathing during sleep (sleep apnoea), severe muscle pain with fever and dark urine (rhabdomyolysis) and ringing in the ears.
- If you have kidney disease and have accidentally taken more tablets than your doctor has prescribed, you may feel drowsy, confused, have hallucinations.
5. What should I know while using Stelax?
Things you should do
Keep all doctor's appointments, so that your progress can be checked.
Call your doctor straight away if you:
- Become pregnant or think you might be pregnant.
Remind any doctor, dentist or pharmacist you visit that you are using Stelax.
Call your doctor straight away or go to a hospital if you have thoughts of harming or killing yourself.
Some people treated with Stelax have had suicidal thoughts. Ask a relative or close friend to tell you if they are worried about any changes in your behaviour.
Things you should not do
- Do not stop using this medicine suddenly unless your doctor tells you to.
- Do not use this medicine to treat any other problems unless your doctor tells you to.
- Do not give this medicine to anyone else. Even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Stelax affects you.
Stelax may cause sleepiness and decreased alertness in some people, especially when you start taking it.
Drinking alcohol
Tell your doctor if you drink alcohol.
Alcohol may make you feel more sleepy when taking Stelax.
Looking after your medicine
- Keep your tablets in the original container until it is time to take them.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
If your doctor tells you to stop taking Stelax or the expiry date on the medicine has passed, ask your pharmacist what to do with any tablets you have left over.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
Side effects happen mainly at the start of treatment with Stelax or if the dose is too high or increased too quickly. Your doctor will adjust your dose if necessary.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Brains and nerves:
| Speak to your doctor if you have any of these less serious side effects and they worry you. If these side effects become severe, tell your doctor, pharmacist or healthcare provider. |
Serious side effects
| Serious side effects | What to do |
Skin or Muscle Problems:
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Stelax contains
| Active ingredient (main ingredient) | Baclofen |
| Other ingredients (inactive ingredients) | Microcrystalline cellulose Maize starch Povidone Colloidal anhydrous silica Magnesium stearate |
| Potential allergens | None |
The tablets are gluten free.
Do not take this medicine if you are allergic to any of these ingredients.
What Stelax looks like
Stelax 10 is a round off-white tablet marked BL│10 on one side, and plain on the other. Bottle of 100 tablets (Aust R 92251).
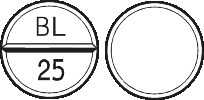
Stelax 25 is a round off-white tablet marked BL│25 on one side, and plain on the other. Bottle of 100 tablets (Aust R 92252).
Who distributes Stelax
Stelax is supplied in Australia by:
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
www.arrotex.com.au
This leaflet was prepared in September 2023.
Published by MIMS November 2023

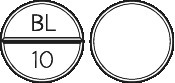
 C10H12ClNO2. Molecular weight: 213.67.
C10H12ClNO2. Molecular weight: 213.67.