What is in this leaflet
This leaflet answers some common questions about Tegretol.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the final page. More recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. You can also download the most up to date leaflet from www.novartis.com.au. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you or your child taking this medicine against the benefits they expect it will have.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Tegretol is used for
Tegretol has several uses:
- to control epilepsy, a condition in which there are repeated seizures (fits). There are many different types of seizures, ranging from mild to severe.
- to control sudden, repeated attacks of facial pain, known as trigeminal neuralgia
- to control mania, a mental condition with episodes of overactivity, elation or irritability
- to control bipolar mood disorder where periods of mania alternate with periods of depression.
Tegretol belongs to a group of medicines called anticonvulsants.
These medicines are thought to work by regulating the way messages in the brain are passed on by nerves so that seizures do not happen. Tegretol also regulates other nerve functions in the body.
Tegretol may be used alone or in combination with other medicines to treat your condition.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another purpose.
Tegretol is only available with a doctor's prescription. There is no evidence that this medicine is addictive.
Before you take Tegretol
When you must not take it
Do not take Tegretol if you have an allergy to:
- carbamazepine (the active ingredient of Tegretol) or any of the other ingredients of Tegretol listed at the end of this leaflet
- any other medicine containing carbamazepine
- tricyclic antidepressants, which are medicines use to treat depression
Tell your doctor if you are allergic to oxcarbazepine, the active ingredient in Trileptal, or to phenytoin. These two medicines are also used to treat epilepsy. Some people who are allergic to oxcarbazepine or phenytoin are also allergic to Tegretol.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, eyelids, throat, mouth, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take Tegretol if you are taking a medicine called a monoamine oxidase inhibitor (MAOI) or have been taking it within the past 14 days. Taking this medicine with a MAOI, or within 14 days of taking a MAOI, may cause a serious reaction with a sudden increase in body temperature, extremely high blood pressure and severe convulsions.
Do not take Tegretol if you have, or have had, any of the following conditions:
- severe liver or heart disease
- a disease of the blood with a reduced number of red or white blood cells or platelets (risk of bone marrow depression)
- an irregular heartbeat caused by a condition called A-V block
- systemic lupus erythematosus, also called SLE
- hepatic porphyria, a disturbance in the production of porphyrin, a pigment important for liver function and blood formation
If you are not sure whether any of the above conditions apply to you, ask your doctor.
Do not take Tegretol after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. In that case, return it to your pharmacist.
If you are not sure whether you should start taking Tegretol, talk to your doctor or pharmacist.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives. Your doctor will want to know if you are prone to allergies.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- heart problems
- glaucoma
- prostate problems or if you cannot retain your urine
- liver or kidney problems
- problems with your blood in the past that were caused by medicines you were taking
- a mental disorder such as depression or schizophrenia
Tell your doctor if you have an intolerance to fructose. In that case, you should not take Tegretol liquid but you can take Tegretol tablets. Each mL of Tegretol liquid contains 175 mg of sorbitol. Sorbitol is converted by the liver to fructose. If people with intolerance to fructose take sorbitol, it can lead to stomach upset and diarrhoea.
Tegretol liquid contains parahydroxybenzoates which may cause allergic reactions.
Tell your doctor if you are of Asian descent, particularly if you are Chinese or Thai. Your doctor may want to do a genetic test before you take Tegretol for the first time.
The risk of serious skin reactions in patients of Han Chinese or Thai origin associated with carbamazepine or chemically-related compounds may be predicted by testing a blood sample of these patients.
Your doctor should be able to advise if a blood test is necessary before taking Tegretol.
Tell your doctor if you are pregnant or intend to become pregnant. You may need to change your medication. Your doctor will discuss with you the potential risk of taking Tegretol during pregnancy since it may cause harm or abnormalities in your baby during pregnancy and soon after birth. Risk of neurodevelopmental disorders (how the brain functions leading to difficulties in social, emotional and mental function) cannot be excluded among children born to women with epilepsy treated with carbamazepine alone or in combination with other antiepileptic drugs during pregnancy. But, if you have epilepsy, it is very important to control your fits while you are pregnant. Your doctor can help you decide whether or not you should take Tegretol in this case.
Tell your doctor if you are breast-feeding or plan to breast-feed. Tegretol passes into breast milk but it is unlikely to affect your baby. With the advice of your doctor, you may breast-feed provided that you watch your baby for any signs of an unwanted side effect. If your baby develops a skin rash, becomes very sleepy or has other unusual symptoms, do not breast-feed again until you speak to your doctor.
If you have not told your doctor about any of these things, tell him/her before you take Tegretol.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Many medicines and Tegretol may interfere with each other. These include:
- MAOI medicines. Tegretol must not be taken together with a MAOI or within 14 days of taking a MAOI
- other medicines used to treat depression such as fluvoxamine, nefazodone, paroxetine, bupropion, citalopram, tricyclic antidepressants and trazodone
- other medicines used to treat seizures including phenytoin, levetiracetam, valproic acid, brivaracetam, lamotrigine, topiramate and oxcarbazepine
- some medicines used to treat mental disorders such as clozapine, haloperidol, thioridazine, lithium, olanzapine, quetiapine, risperidone and ziprasidone
- some medicines used to treat heart problems or high cholesterol
- some medicines used to help you sleep or calm you down
- some pain relievers such as paracetamol, dextro-propoxyphene, tramadol and ibuprofen
- warfarin, a medicine used to prevent blood clots
- some diuretics (fluid tablets), which are medicines used to reduce water retention and high blood pressure
- some antibiotics and antifungal medicines used to treat infections, such as erythromycin, clarithromycin, doxycycline, itraconazole, ketoconazole, fluconazole, voriconazole and rifampicin
- corticosteroids such as prednisolone and dexamethasone
- St John's wort, an ingredient in many medicines that you can buy without a prescription from a pharmacy, health food shop or supermarket.
- antihistamines such as loratadine and terfenadine, which are medicines used to prevent or relieve the symptoms of allergies such as hay fever
- isoniazid, a medicine used to prevent and treat tuberculosis
- acetazolamide, a medicine used to reduce fluid retention and to treat glaucoma and some types of seizures
- medicines used to treat stomach or duodenal ulcers, such as cimetidine and omeprazole
- muscle relaxants such as dantrolene and oxybutynin
- ticlopidine, a medicine used to prevent blood clotting
- some medicines used to treat asthma, such as theophylline and aminophylline
- some medicines used to prevent rejection of organ transplants and to treat severe rheumatoid arthritis and some skin diseases, such as cyclosporin and everolimus
- some medicines used to treat cancer, such as cisplatin, doxorubicin and imatinib
- methadone, a medicine used to control severe pain and to treat heroin addiction
- metoclopramide, a medicine used to treat nausea and vomiting
- isotretinoin, a medicine used to treat acne
- danazol, a medicine used to treat endometriosis
- a vitamin called nicotinamide
- medicines used to treat HIV such as indinavir, ritonavir and saquinavir
- levothyroxine, a medicine used to treat underactive thyroids
- praziquantel, a medicine used to treat worm infections of the blood
- medicines containing oestrogen and progesterone, including hormone replacement therapy and contraceptives
The above medicines may be affected by Tegretol or they may affect how well it works. You may need to take different amounts of your medicines or you may need to take different medicines.
Tell your doctor if you are using hormonal contraceptives (e.g. birth control pills or injections). If you are a female of childbearing age you should use an effective method of contraception throughout your treatment and for 2 weeks after your last dose. If you begin taking Tegretol while you are using hormonal contraceptives, they may not work as well as they should. Unplanned pregnancies can happen. Your doctor can suggest another form of birth control (non-hormonal) while you are taking Tegretol.
Tell your doctor immediately if you notice irregular vaginal bleeding or spotting.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking Tegretol.
How to take Tegretol
Follow all directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much Tegretol you need to take each day. This may depend on your age, your medical condition and whether or not you are taking other medicines.
Your doctor will usually start your treatment with a low dose and then slowly increase it to the lowest amount needed to control your condition. Some people will need higher doses than other people will.
How to take it
Tegretol is available in conventional tablets, controlled release (CR) tablets and in liquid form. The liquid is usually used for children or adults who have trouble swallowing tablets.
If you are taking Tegretol tablets, swallow them with a full glass of water.
If you are taking Tegretol CR tablets, do not crush or chew them. The CR tablets have a special coating that would be destroyed by crushing or chewing the tablet.
If the dose is one-half tablet, you can buy a tablet cutter from your pharmacist to make sure the dose is accurate.
If you are taking Tegretol liquid, shake the bottle well before each dose is measured. Shaking the bottle and using a medicine measure will make sure that you take the correct dose. You can get a medicine measure from your pharmacist.
When to take it
Take your dose of Tegretol during or after a meal. This helps to prevent stomach upset.
Tegretol is usually taken in 2 or 3 doses during the day. But your doctor may tell you to take it more or less often, depending on your situation.
If you forget to take it
If your next dose is not due for more than 2 or 3 hours, take the missed dose as soon as you remember. Then take your next dose at the usual time and continue on with your normal schedule.
If your next dose is due within 2 or 3 hours, skip the missed dose. Take your next dose at the usual time and continue on with your normal schedule.
Do not take a double dose to make up for the one that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Tegretol helps to control your condition but does not cure it. You must take it every day, even if you feel well.
Do not stop taking Tegretol or lower the dose without first checking with your doctor. Do not let yourself run out of medicine over the weekend or on holidays. Stopping your medicine suddenly or lowering the dose may cause unwanted side effects or make your condition worse. If you are taking this medicine to treat epilepsy, you could develop seizures (fits). Your doctor will usually reduce the dose slowly before you can stop taking it completely.
If you take too much (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much Tegretol. Do this even if there are no signs of discomfort or poisoning.
Keep the telephone numbers for these places handy.
Some of the symptoms of an overdose may include agitation, disorientation, fainting, vomiting, difficulty breathing, fast and irregular heartbeat, blurred vision, shakiness and slurred speech. If you are taking the controlled release (CR) tablets, it may take longer for you to notice these effects.
While you are taking Tegretol
Things you must do
If you become pregnant while taking Tegretol, tell your doctor immediately. Your doctor can discuss with you the risks of taking it while you are pregnant.
Be sure to keep all of your doctor's appointments so that your progress can be checked.
To help prevent unwanted side effects from happening, your doctor may want to do some tests before you start taking Tegretol and from time to time during the course of your treatment.
Contact your doctor immediately if at any time you have thoughts of harming or killing yourself. Some people being treated with antiepileptics have had thoughts of harming or killing themselves. All thoughts of suicide must be taken seriously.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise your doctor may think that it was not effective and change your treatment unnecessarily.
Before having any surgery or emergency treatment, tell the doctor or dentist in charge that you are taking Tegretol. This medicine may interfere with some of the medicines used during surgery.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Tegretol.
Tell any other doctor, dentist or pharmacist who treats you that you are taking Tegretol.
Things you must not do
Do not stop taking Tegretol or lower the dose without first checking with your doctor.
Do not use Tegretol to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours or they have the same condition as you do.
Things to be careful of
Avoid drinking grapefruit juice while you are being treated with Tegretol. Grapefruit juice may interact with Tegretol and affect how your body uses this medicine.
Be careful driving, operating machinery or doing jobs that require you to be alert until you know how Tegretol affects you. Children should avoid doing things like riding bicycles or climbing trees. This medicine may cause dizziness, drowsiness, blurred vision, double vision or lack of muscle coordination in some people, especially when you first start to use it or when the dose is increased.
Be careful when drinking alcohol while you are taking Tegretol. The combination could make you more sleepy, dizzy or light headed than usual. Your doctor may suggest you avoid alcohol while you are being treated with Tegretol.
When outdoors, wear protective clothing and use at least a 15+ sunscreen. Do not use a sunlamp or tanning bed or booth. This medicine may cause your skin to be much more sensitive to sunlight than it normally is. Exposure to sunlight may cause a skin rash, itching, redness or severe sunburn. If your skin does appear to be burning, tell your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Tegretol.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects. If you are over 65 years old, you may have an increased risk of getting side effects.
Do not be alarmed by the following lists of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following side effects and they worry you:
- dizziness or light headedness
- tiredness or drowsiness
- eye pain
- weakness, unsteadiness when walking
- headache
- restlessness, agitation or confusion
- difficulty in speaking or slurred speech
- numbness or tingling in hands or feet
- muscle pain or cramps
- nausea (feeling sick) or vomiting, loss of appetite
- weight gain
- stomach pain or discomfort
- diarrhoea
- constipation
- dry mouth
- swollen, red, sore tongue
- mouth ulcers or cold sores
- change in sense of taste
- blurred or double vision, swollen runny eyes, difficulty seeing
- ringing or buzzing in the ears or other changes in hearing
- frequent need to urinate (pass water)
- sweating
- hair loss
- acne
- change in skin colour
- excessive hairiness, especially in women
- sexual disturbances such as impotence
- breast enlargement in men
- unusual secretion of breast milk
- loss of muscle coordination
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- signs of allergy such as swelling of the face, lips, tongue, eyelids, throat, mouth or other parts of the body; wheezing or troubled breathing, difficulty swallowing, itching, hives, chest discomfort or tightness, loss of consciousness
- skin rash, redness, blisters or peeling skin, accompanied by fever, chills, headache, cough, body aches
- sudden increase in body temperature, accompanied by sweating, fast heart beat and muscle stiffness, altered consciousness, high blood pressure, excessive salivation
- constant "flu-like" symptoms (chills, fever, sore throat, swollen glands, aching joints, lack of energy)
- unusual bleeding or bruising under the skin, nosebleeds
- shortness of breath and dizziness when exercising
- frequent infections or fever
- severe chills, sore throat, swollen glands or mouth ulcers
- persistent nausea or vomiting, loss of appetite and feeling generally unwell, which may be accompanied by pain in the abdomen, fever, itching, a yellow colour to skin or eyes, dark coloured urine or light coloured bowel motions
- diarrhoea, abdominal pain and fever
- severe upper stomach pain, often with loss of appetite and vomiting
- more frequent or more severe seizures (fits)
- sudden onset of uncontrollable muscle spasms affecting the eyes, head, neck and body
- trembling, uncontrolled body movements
- lethargy, confusion
- depression, aggressive behaviour, recurrence of a previous mental illness, hallucinations (seeing or hearing things that aren't there)
- swelling of the feet and legs or weight increase due to fluid build-up
- changes in behaviour, weakness
- change in heartbeat (fast, slow, irregular), sometimes with fainting or chest pain
- passing less urine than normal which may be accompanied by lack of energy, vomiting, headache and confusion
- blood in the urine
- symptoms of sunburn such as redness, itching, swelling or blistering that may happen more quickly than normal
- red blotchy rash mainly on the face which may be accompanied by fatigue, fever, nausea, loss of appetite
- swelling and redness along a vein or nerve, which is extremely tender when touched
- signs that blood clots may have formed, such as sudden severe headache, sudden loss of coordination or vision, pain in the calves, thighs or chest
- severe headache accompanied by stiff neck, muscle spasms and extreme sensitivity to bright light
- a fall due to dizziness, drowsiness, decrease in blood pressure or confusion.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may happen in some people. Some of these side effects (for example, changes in sodium levels, thyroid function, structure of bones, cholesterol level or blood pressure) can only be found when your doctor does tests from time to time to check your progress.
After taking Tegretol
Storage
- Keep your medicine in the original container until it is time to take it.
- Store the medicine in a cool dry place.
- Do not store Tegretol or any other medicine in the bathroom or near a sink.
- Do not leave it in the car or on window sills.
Heat and dampness can destroy some medicines. Tegretol will keep well if it is cool and dry.
Keep Tegretol where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Tegretol or you find that it has passed the expiry date, ask your pharmacist what to do with any medicine you have left over.
Product description
What it looks like
Tegretol tablets:
- Tegretol 100 mg: white tablets marked with a break line and BW on one side, GEIGY on the other side; packs of 100 tablets.
- Tegretol 200 mg: white tablets marked with a break line and GK on one side, CG on the other side; packs of 100 tablets.
Tegretol CR tablets:
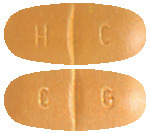
- Tegretol CR 200 mg: beige- orange capsule shaped tablets with a break line on both sides, marked H/C on one side and C/G on the other; in PVC/PE/PVDC/Al blister packs of 100 or 200 tablets.
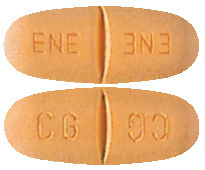
- Tegretol CR 400 mg: brown- orange capsule shaped tablets with a break line on both sides, marked ENE/ENE on one side and CG/CG on the other; in PVC/PE/PVDC/Al blister packs of 100 or 200 tablets.
Tegretol liquid:
- a thick, white, caramel flavoured liquid, packed in 300 mL brown glass bottles with a child-resistant cap.
Ingredients
Tegretol tablets contain 100 mg or 200 mg of carbamazepine as the active ingredient. They also contain:
- cellulose-microcrystalline
- carmellose sodium
- silica-colloidal anhydrous
- magnesium stearate
Tegretol CR tablets contain 200 mg or 400 mg of carbamazepine as the active ingredient. They also contain:
- cellulose-microcrystalline
- silica-colloidal anhydrous
- magnesium stearate
- talc
- Aquacoat ECD-30
- acrylates copolymer
- carmellose sodium
- hypromellose
- polyoxyl 40 hydrogenated castor oil
- iron oxide red CI 77491
- iron oxide yellow CI 77492
- titanium dioxide
Tegretol liquid contains 100 mg of carbamazepine per 5 mL of liquid. It also contains:
- methyl hydroxybenzoate
- propyl hydroxybenzoate
- caramel flavour
- propylene glycol
- PEG-8 stearate
- saccharin sodium
- sorbic acid
- sorbitol solution (70%)
- cellulose-dispersible
- hyetellose
- purified water
Diabetic patients can take Tegretol liquid. It contains sorbitol solution (875 mg/ 5 mL) which is slowly converted into glucose, providing 14 kJ per 5 mL of liquid. Tegretol liquid contains traces of benzoates.
Sponsor
Tegretol is supplied in Australia by:
NOVARTIS Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1-800-671-203
® = Registered Trademark
This leaflet was prepared in December 2023
Australian Registration Number.
Tegretol 100 mg AUST R 41846
Tegretol 200 mg AUST R 41848
Tegretol CR 200 mg AUST R 42974
Tegretol CR 400 mg AUST R 42944
Tegretol Liquid AUST R 59160
(tgr051223c) based on PI (tgr051223i)
Published by MIMS January 2024



 The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
 Chemical Name: 5H-dibenzo[b,f]azepine-5-carboxamide.
Chemical Name: 5H-dibenzo[b,f]azepine-5-carboxamide.