SUMMARY CMI
Temozolomide Alphapharm
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using Temozolomide Alphapharm?
Temozolomide Alphapharm contains the active ingredient Temozolomide. Temozolomide Alphapharm is used to treat patients with brain tumours. Temozolomide Alphapharm is also used to treat adult patients with advanced metastatic malignant melanoma.
For more information, see Section 1. Why am I using Temozolomide Alphapharm? in the full CMI.
2. What should I know before I use Temozolomide Alphapharm?
Do not use if you have ever had an allergic reaction to Temozolomide Alphapharm or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Temozolomide Alphapharm? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Temozolomide Alphapharm and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Temozolomide Alphapharm?
- Your doctor has worked out the exact dose of Temozolomide Alphapharm for you according to your individual needs.
- You may be given other medication to take before or after Temozolomide Alphapharm to help stop nausea.
- Take Temozolomide Alphapharm without food at least one hour before a meal.
- It is good practice to take Temozolomide Alphapharm at about the same time each day.
More instructions can be found in Section 4. How do I use Temozolomide Alphapharm? in the full CMI.
5. What should I know while using Temozolomide Alphapharm?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Temozolomide Alphapharm? in the full CMI.
6. Are there any side effects?
Serious side effects may include: shortness of breath; tingling or numbness in hands or feet; bruising, bleeding or being unusually pale or tired. This could be caused by a low level of platelets or red blood cells in the blood; shivering that is associated with chills and fever. This could be sign of an infection caused by a low level of white blood cells in the blood; symptoms such as fever, headache, personality change, seizures and/or vomiting which could be associated with a brain infection caused by a herpes virus; new or recurring cytomegalovirus infection and return of hepatitis B; symptoms of diabetes, such as passing large amounts of urine and constant thirst; development of red or purple spots under the skin; symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin. Nausea, tiredness, dark urine and yellowing of the skin which could be symptoms of hepatotoxicity.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
Temozolomide Alphapharm
Active ingredient(s): Temozolomide
Consumer Medicine Information (CMI)
This leaflet provides important information about using Temozolomide Alphapharm. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Temozolomide Alphapharm.
Where to find information in this leaflet:
1. Why am I using Temozolomide Alphapharm?
2. What should I know before I use Temozolomide Alphapharm?
3. What if I am taking other medicines?
4. How do I use Temozolomide Alphapharm?
5. What should I know while using Temozolomide Alphapharm?
6. Are there any side effects?
7. Product details
1. Why am I using Temozolomide Alphapharm?
Temozolomide Alphapharm contains the active ingredient Temozolomide. Temozolomide Alphapharm belongs to a group of medicines called cytotoxic or chemotherapy medicines. Temozolomide Alphapharm works by killing cancer cells and stopping cancer cells from growing and multiplying.
Temozolomide Alphapharm is used to treat patients with brain tumours. Temozolomide Alphapharm is also used to treat adult patients with advanced metastatic malignant melanoma.
Your doctor, however, may prescribe Temozolomide Alphapharm for another purpose. Ask your doctor if you have any questions about why Temozolomide Alphapharm has been prescribed for you.
Use in children: Temozolomide Alphapharm capsules are used to treat children 3 years and older, with specific forms of brain tumour (glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy).
This medicine is only available with a doctor's prescription.
2. What should I know before I use Temozolomide Alphapharm?
Warnings
Do not use Temozolomide Alphapharm if:
- you are allergic to Temozolomide Alphapharm (temozolomide), dacarbazine (DTIC) or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
Symptoms of an allergic reaction may include:
- hives, itching or skin rash
- swelling of the face, lips or tongue. This may lead to difficulty swallowing.
- shortness of breath, wheezing, difficulty breathing or a tight feeling in your chest.
- you or your partner are pregnant or intend to become pregnant.
- you are breastfeeding.
- you have a very low level of white blood cells, red blood cells or platelets.
- the expiry date has passed or the packaging is torn, damaged or shows signs of tampering.
Check with your doctor if you:
- vomit frequently. Your doctor may give you medicine to control the vomiting
- are anaemic or have blood clotting problems
- intend to have children. Temozolomide Alphapharm may cause infertility in men
- have liver or kidney problems
- have viruses such as cytomegalovirus and hepatitis B
- have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes - take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Temozolomide Alphapharm may cause birth defects if either the male or female is using Temozolomide Alphapharm at the time of conception or during pregnancy. Therefore, female patients must have a negative pregnancy test before starting Temozolomide Alphapharm. Both male and female patients and their partners should each use some kind of birth control while taking Temozolomide Alphapharm. Male patients whose partners are already pregnant should use a condom to minimise exposure of the unborn baby to Temozolomide Alphapharm in the sperm.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Temozolomide Alphapharm and affect how it works. These include other medicines used to treat cancer or any other treatment that may lower your immune system, and valproic acid, used to treat epilepsy and bipolar disorder. You may need different amounts of your medicine or you may need to use different medicines. Your doctor will advise you.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Temozolomide Alphapharm.
4. How do I use Temozolomide Alphapharm?
How much to take
- Follow all directions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
- Your doctor has worked out the exact dose of Temozolomide Alphapharm for you according to your individual needs.
- You may be given other medication to take before or after Temozolomide Alphapharm to help stop nausea.
- If you are taking Temozolomide Alphapharm in combination treatment with radiation (newly diagnosed patients):
If you are a patient with a newly diagnosed brain tumour, your doctor will start you on a dose of Temozolomide Alphapharm every day for 42 days (up to 49 days) in combination with radiation therapy. This is the first part of the treatment ("concomitant phase") in which you complete the radiation therapy. Your treatment will be interrupted for 4 weeks to give your body a chance to recover.
Then, you will start the next phase of treatment ("adjuvant phase") and your Temozolomide Alphapharm dose will change. In this phase, there are up to 6 treatment cycles. Each treatment cycle lasts 28 days. You will take your new dose of Temozolomide Alphapharm capsules once daily for the first 5 days ("dosing days") of each cycle, followed by 23 days without Temozolomide Alphapharm; this adds up to a 28 day treatment cycle. After day 28, the next cycle will begin, in which you will again take this medicine once daily for 5 days followed by 23 days without Temozolomide Alphapharm. Before each new treatment cycle begins, your blood will be tested to determine if the Temozolomide Alphapharm dose needs to be adjusted.
- If you are taking only Temozolomide Alphapharm (patients treated for recurrent brain tumour):
Take the dose the doctor has prescribed once a day for 5 days. Depending on your response to Temozolomide Alphapharm, a new treatment cycle will begin each 28 days. You will then take this medicine again once daily for 5 days. Before each new treatment cycle, your blood will be tested to see if the dose needs to be changed. - Each time you start a new treatment cycle, be sure you understand exactly how many capsules of each strength you need to take on each day of dosing.
- All patients: Temozolomide Alphapharm comes in different strength capsules (shown on the outer label in mg). Each strength is a different colour. Depending on the dose of Temozolomide Alphapharm that your doctor prescribes, you may have to take several capsules on each dosing day of the treatment cycle.
Be sure you understand exactly how many capsules you need to take of each strength. Ask your doctor or pharmacist to write down the number of each strength (include colour) that you need to take on each dosing day.
- Be sure you know exactly which days are your dosing days.
- Be sure you review the dose with your health care provider each time you start a new cycle. Sometimes the dose or the mix of capsules you need to take will be different from the last cycle. - Once you take the medicine home, if you are confused or unsure about how to take your dose, call for reinstruction before beginning the treatment cycle. Errors in how you take this medicine may have serious health consequences.
- Swallow the capsules whole with a glass of water. Do not open or chew the capsules.
When to take Temozolomide Alphapharm
- Take Temozolomide Alphapharm without food at least one hour before a meal.
- It is good practice to take Temozolomide Alphapharm at about the same time each day.
- If vomiting occurs after you take your capsules, do not take another dose that day.
- Your doctor will tell you when your treatment should be stopped.
If you forget to take Temozolomide Alphapharm
Temozolomide Alphapharm should be used regularly at the same time each day. If you miss a dose, take the missed dose as soon as possible during the same day. If a full day has gone by, check with your doctor.
Do not double the next dose unless your doctor tells you to do so.
If you take too much Temozolomide Alphapharm
If you think that you have used too much Temozolomide Alphapharm, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre (by calling 13 11 26),
or - contact your doctor,
or - go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Temozolomide Alphapharm?
Things you should do
Call your doctor straight away if you:
- Feel sick or vomit while being treated with Temozolomide Alphapharm. Your doctor may give you another medicine to help stop this.
- Become unusually pale or tired, get blood clotting problems or frequent infections while being treated with Temozolomide Alphapharm. These could be caused by a low level of red blood cells, platelets or white blood cells in the blood. This is more common in patients over 70 years of age. Your doctor may need to change your dose of Temozolomide Alphapharm.
- Or your partner becomes pregnant while you are being treated with Temozolomide Alphapharm.
Remind any doctor, dentist or pharmacist you visit that you are using Temozolomide Alphapharm.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Be sure to keep all your doctor's appointments so your progress can be checked.
Your doctor may need to do some blood and other tests from time to time to check on your progress and detect any unwanted side effects.
Keep follow up appointments with your doctor.
It is important to have your follow-up doses of Temozolomide Alphapharm at the appropriate times to get the best effects from your treatment.
If you are about to be started on any new medicine, tell your doctor, dentist or pharmacist that you are being treated with Temozolomide Alphapharm.
Things you should not do
- Do not open the capsules. If a capsule is damaged, avoid contact with your skin, eyes and nose. Avoid inhaling the powder. If you touch the powder or get some in your eyes or nose, wash the area with water.
- Do not give Temozolomide Alphapharm to anyone else, even if they have the same condition as you.
- Do not use Temozolomide Alphapharm to treat any other complaints unless your doctor tells you to.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Temozolomide Alphapharm affects you. As with other medicines, Temozolomide Alphapharm may make some people feel tired. If this occurs do not drive.
Looking after your medicine
- Keep your Temozolomide Alphapharm capsules in the bottle until it is time to use them.
- Keep Temozolomide Alphapharm in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Temozolomide Alphapharm.
Like other medicines that treat cancer, Temozolomide Alphapharm may have unwanted side effects. Sometimes they may be serious, most of the time they are not. You may need medical attention if you get some of these side effects.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
These are the more common side effects of Temozolomide Alphapharm.
Serious side effects
| Serious side effects | What to do |
| Tell your doctor as soon as possible if you notice any of these serious side effects. |
Shivering associated with chills and fever or the development of red or purple spots under the skin may take some time to occur. Therefore, even after you have finished your treatment with Temozolomide Alphapharm, you should tell your doctor immediately if you notice these side effects.
Very serious side effects
| Very serious side effects | What to do |
| Symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin | Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of these very serious side effects. |
Other side effects not listed above may also occur in some patients.
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Temozolomide Alphapharm contains
| Active ingredient (main ingredient) | temozolomide |
| Other ingredients (inactive ingredients) | lactose (in the 5mg and 20mg capsules only), sodium starch glycollate, stearic acid, tartaric acid, microcrystalline cellulose, colloidal anhydrous silica.
|
Temozolomide Alphapharm does not contain sucrose, gluten, tartrazine or other azo dyes.
Do not take this medicine if you are allergic to any of these ingredients.
What Temozolomide Alphapharm looks like
The 5 mg capsules are light green opaque, marked with black imprint "TMZ 5 mg". (AUST R 192687)
The 20 mg capsules are rich yellow marked with black imprint "TMZ 20 mg". (AUST R 192678)
The 100 mg capsules are flesh coloured, marked with black imprint "TMZ 100 mg". (AUST R 192684)
The 140 mg capsules are powder blue marked with black imprint "TMZ 140 mg". (AUST R 192680)
The 180 mg* capsules are orange marked with black imprint "TMZ 180 mg". (AUST R 192692)
The 250 mg capsules are buff coloured marked with black imprint "TMZ 250 mg". (AUST R 192681)
*The 180mg capsules are not marketed.
Tell your pharmacist if you notice any change in the appearance of the capsule.
Who distributes Temozolomide Alphapharm
Alphapharm Pty Ltd (Mylan Australia)
Cnr Garnet and Antimony Streets
Carole Park, QLD 4300
Australia
This leaflet was prepared in February 2020.
Published by MIMS February 2020

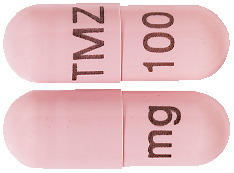
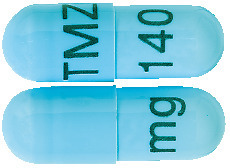







 In clinical trials, the most frequently occurring undesirable effects were gastrointestinal disturbances, specifically nausea (43%) and vomiting (36%). These effects were usually Grade 1 or 2 (mild to moderate in severity) and were either selflimiting or readily controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%. Severe myelosuppression, predominantly thrombocytopenia, was dose-limiting and occurred in 7% of all patients. Anaemia was reported in 5% of patients. Severe neutropenia and leucopenia occurred in 3% and 2% of patients, respectively.
In clinical trials, the most frequently occurring undesirable effects were gastrointestinal disturbances, specifically nausea (43%) and vomiting (36%). These effects were usually Grade 1 or 2 (mild to moderate in severity) and were either selflimiting or readily controlled with standard anti-emetic therapy. The incidence of severe nausea and vomiting was 4%. Severe myelosuppression, predominantly thrombocytopenia, was dose-limiting and occurred in 7% of all patients. Anaemia was reported in 5% of patients. Severe neutropenia and leucopenia occurred in 3% and 2% of patients, respectively.

