SUMMARY CMI
TYKERB®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about taking this medicine, speak to your doctor or pharmacist.
1. Why am I taking TYKERB?
TYKERB contains the active ingredient lapatinib. TYKERB is used to treat breast cancer that may have spread to other parts of your body. For more information, see Section 1. Why am I taking TYKERB? in the full CMI.
2. What should I know before I take TYKERB?
Do not use if you have ever had an allergic reaction to TYKERB or any of the ingredients listed at the end of the CMI. TYKERB may also be used with other medicines including trastuzumab, paclitaxel, capecitabine or other medicines called "aromatase inhibitors" so it is important to read the Consumer Medicine Information (CMI) for these medicines as well.
Talk to your doctor if you have any other medical conditions affecting your heart, lungs, or liver. Your doctor will want to monitor these closely and will send you for regular blood tests to check how you are responding to treatment. Tell your doctor if you take any other medicines or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take TYKERB? in the full CMI.
3. What if I am taking other medicines?
Some medicines interfere with TYKERB and affect how it works. Grapefruit in any form will also affect how well TYKERB works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take TYKERB?
- Your doctor will decide how much TYKERB you will need to take. This may change depending on how your body responds or whether you are taking any additional medicines for your breast cancer.
- TYKERB must be taken on an empty stomach at least 1 hour before or 1 hour after eating.
More instructions can be found in Section 4. How do I take TYKERB? in the full CMI.
5. What should I know while taking TYKERB?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking TYKERB? in the full CMI.
6. Are there any side effects?
Common side effects include feeling sick, dizzy, or tired, weight loss, diarrhoea, tummy pain, sore throat, dry, cracked skin/nails, hair loss, nose bleeds, feeling sore, sore/red mouth, back pain, trouble sleeping, rash, red/irritated and itchy skin. More serious side effects include trouble breathing, wheezing, fainting, chest pain, blue lips or skin, yellowing of skin or eyes, blistering or swelling of skin, eyes, lips or tongue, changes to heart rate, severe tummy pain/diarrhoea, tingling or numbness in hands, feet or limbs, trouble doing a wee; cracked lips and skin while feeling very thirsty, weakness, chills, pale skin, being more sick than usual.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
TYKERB® (TIE-curb)
Active ingredient(s): lapatinib (La·pa·tee-nib)
Consumer Medicine Information (CMI)
This leaflet provides important information about taking TYKERB. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about taking TYKERB.
Where to find information in this leaflet:
1. Why am I taking TYKERB?
2. What should I know before I take TYKERB?
3. What if I am taking other medicines?
4. How do I take TYKERB?
5. What should I know while taking TYKERB?
6. Are there any side effects?
7. Product details
1. Why am I taking TYKERB?
TYKERB contains the active ingredient lapatinib. TYKERB is a medicine known as a 'protein kinase inhibitor' meaning when used with other medicines, it may slow or stop the spread of cancer cells that have originated from your breast.
TYKERB is used with other medicines to treat breast cancer that may or may not yet have spread to other parts of your body. When cancer cells spread to other parts of your body (a process called "metastasis") they may be growing because of hormones (chemicals) that are naturally produced in your body such as oestrogen and progesterone.
2. What should I know before I take TYKERB?
Warnings
Do not take TYKERB if:
- You are allergic to lapatinib, or any of the ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can use this medicine.
- The expiry date has passed on the packaging or shows evidence of tampering.
- You are under 18 years of age
Some signs of an allergic reaction may include difficulty breathing or shortness of breath leading to pale or blue lips/skin. Other signs can include swelling of the lips, face, or tongue as well as skin problems including rash, hives or painful and itchy red skin that are sudden and spreading.
Check with your doctor if you:
- have a heart condition for example an irregular heartbeat
- have any lung problems or breathing difficulties
- have a liver condition
- are taking other medicines to treat infections (such as ketoconazole, itraconazole, rifampin) or epilepsy (carbamazepine, phenytoin). See section "what if I am taking other medicines"
- have any allergies to food, preservatives, dyes or other medicines
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Monitoring
Your doctor may want to monitor your heart and lungs while you are on treatment with TYKERB.
Your doctor may also send you for blood tests before starting treatment or once you have started treatment to monitor your liver function.
These blood tests may also be looking for the number of white blood cells in your body as TYKERB when used with some medicines may reduce the number of white blood cells in your body. This can make you more likely to get sick.
Your doctor may alter your treatment plan depending on your results.
Changes to toilet habits
Tell your doctor immediately if you have noticeable changes to your toilet habits including severe diarrhoea or if you are unable to go to the toilet at all. Your doctor may choose to stop TYKERB treatment if the problem persists or prescribe you with additional medicines to treat diarrhoea.
Skin reactions
Tell your doctor immediately if you have a rash on your skin that is spreading/getting worse. Your doctor may choose to stop treatment with TYKERB based on this.
Birth control
You should avoid becoming pregnant while taking TYKERB. TYKERB may harm your unborn baby.
You must use a reliable form of birth control (contraception) during treatment with TYKERB and for at least 5 days after your last dose. Your doctor can provide you advice on the most effective method.
Taking TYKERB with other medicines
TYKERB is generally taken with other medicines for cancer including trastuzumab, paclitaxel, capecitabine or other medicines called "aromatase inhibitors" (see section "What if I am taking other medicines"). You should also read the "Consumer Medicine Information" for these medicines carefully.
Taking TYKERB with food
TYKERB must be taken on an empty stomach at least 1 hour before OR 1 hour after eating.
You should not drink grapefruit juice or eat grapefruit during your treatment with TYKERB. It may make this medicine less effective and possibly increase the chance of side effects.
Pregnancy and breastfeeding
Tell your doctor straight away if you are pregnant or think you may be pregnant. Talk to your doctor before starting treatment with TYKERB if you intend to become pregnant.
Do not breastfeed while taking TYKERB and for at least 5 days after stopping treatment with TYKERB as it may harm your baby.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins, or supplements that you buy without a prescription from your pharmacy, supermarket, or health food shop.
Taking TYKERB with a medicine called 'trastuzumab'
Trastuzumab is given to you in a hospital and can also be used to treat breast cancer where the cancer may have spread to other parts of your body. Your doctor may consider your dose of TYKERB carefully if you are taking trastuzumab.
Taking TYKERB with a medicine called 'paclitaxel'
Paclitaxel is given to you in a hospital and can also be used to treat breast cancer where the cancer may have spread to other parts of your body. TYKERB may increase the effect of paclitaxel on your body. When taken together, it may cause more severe diarrhoea and changes to your blood test results.
Your doctor may consider your dose of TYKERB carefully if you are also taking paclitaxel.
Taking TYKERB with a medicine called 'capecitabine'
Capecitabine can also be used to treat breast cancer where the cancer may have spread to other parts of your body. Your doctor may consider your dose of TYKERB carefully if you are also taking capecitabine.
Taking TYKERB with medicines called 'aromatase inhibitors'
Aromatase inhibitors (such as medicines containing 'letrozole') can also be used to treat breast cancer that may have spread to other parts of your body. Your doctor may consider your dose of TYKERB carefully if you are also taking letrozole or any other similar medicine.
Other medicines that may affect how well TYKERB works
- Erythromycin, ketoconazole, itraconazole, posaconazole, voriconazole, rifabutin, rifampicin, telithromycin (used to treat infections).
- Ritonavir, saquinavir (used to treat HIV).
- Cisapride (used to treat digestive system problems).
- Esomeprazole or other drugs that decrease stomach acidity (used to treat stomach ulcers or indigestion).
- Medicines used for sedation before surgery (anaesthesia), such as midazolam.
- Quinidine, digoxin (used to treat heart problems)
- Verapamil (used to treat high blood pressure or angina)
- Rosuvastatin (used to treat high cholesterol)
- Repaglinide (used to treat diabetes)
- Phenytoin, carbamazepine (used to treat seizures)
- Pimozide (used to treat mental health problems)
- Nefazodone (used to treat depression)
- St John's Wort (herb extract used to treat depression)
- Cyclosporin (used to prevent the rejection of transplanted organs)
- Topotecan, irinotecan, docetaxel (used to treat cancer).
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect TYKERB.
4. How do I take TYKERB?
How much TYKERB to take
- Follow all instructions from your doctor.
- Your doctor will decide how much TYKERB you need to take. Depending on how well you respond to treatment or if you have any side effects, your doctor may change your dose or stop treatment.
- You may be taking other medicines for your breast cancer in addition to TYKERB. See below for the usual amount of TYKERB you would be taking in those settings.
Taking TYKERB with a medicine called 'trastuzumab'
- The usual amount is 4 (four) TYKERB tablets taken once a day continuously (a total daily dose of 4 x 250 mg tablets is 1000 mg a day) along with the amount of trastuzumab your doctor tells you to.
Taking TYKERB with a medicine called 'paclitaxel'
- The usual amount is 6 (six) TYKERB tablets taken once a day continuously (a total daily dose of 6 x 250 mg tablets is 1500 mg each day) along with the amount of paclitaxel your doctor tells you to.
Taking TYKERB with a medicine called 'capecitabine'
- The usual amount is 5 (five) TYKERB tablets taken once a day continuously (a total daily dose of 5 x 250 mg tablets which is 1250 mg each day) along with the amount of capecitabine your doctor tells you to.
Taking TYKERB with other medicines known as 'aromatase inhibitors'
- The usual amount is 6 (six) TYKERB tablets taken once a day continuously (a total daily dose of 6 x 250 mg tablets is 1500 mg each day) along with the amount of aromatase inhibitor your doctor tells you to.
Your doctor will tell you how much trastuzumab, paclitaxel, capecitabine, or aromatase inhibitor you should take and when you should take it. These medicines may have a different schedule to TYKERB that you will also need to follow.
How to take TYKERB
- TYKERB must be taken on an empty stomach at least 1 hour before OR 1 hour after eating.
- TYKERB tablets need to be swallowed whole with a glass of water. You must not chew, crush, or split the tablets before you swallow it.
When to take TYKERB
- Take TYKERB at the same time every day to help you remember to take it regularly.
- TYKERB must be taken at least one hour before or after you eat.
How long to take TYKERB
- Keep taking TYKERB until your doctor tells you to stop.
If you forget to take TYKERB
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you take too much TYKERB
If you think that you have used too much TYKERB, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while taking TYKERB?
Things you should do
- Attend all your appointments with your doctor as well as any other ones where your heart, lungs or kidneys are being checked.
- Get your blood tests done promptly so your doctor can check how your body is responding to TYKERB.
- Continue taking TYKERB as well as any other medicines you may be taking as directed by your doctor for as long as they tell you to.
- Use effective birth control to avoid becoming pregnant (see section on "Pregnancy and breastfeeding").
- Make sure you take TYKERB on an empty stomach. At least one hour before OR 1 hour after eating.
Call your doctor straight away if you:
- Are pregnant or think you may be pregnant.
- Are experiencing a sudden angry-looking rash, hives or blisters on your skin that is spreading.
- Are experiencing check pain, trouble breathing or shortness of breath.
- Are experiencing noticeable changes to your toilet habits.
- Are experience swelling of your lips, tongue or face
Remind any doctor, dentist, or pharmacist you visit that you are taking TYKERB.
Things you should not do
- Do not drink grapefruit juice or eat grapefruits while you are taking TYKERB.
- Do not give this medicine to someone else.
- Do not stop taking this medicine suddenly.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how TYKERB affects you.
TYKERB can cause tiredness and may make you unfit to drive.
Drinking alcohol
Tell your doctor if you drink alcohol.
Looking after your medicine
- Keep TYKERB tablets in its original packaging until it is time to use it.
- Store in a cool, dry place below 30°C.
Do not store TYKERB in the bathroom or near a sink. Do not store in the car or on a windowsill.
Keep it where young children cannot reach it.
When to discard your medicine
You must only discard this medicine if your Doctor tells you to stop taking TYKERB or if the expiry date on the packaging has passed.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Tummy problems:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Breathing problems
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. As you will likely be taking TYKERB with another medicine you may experience effects related to TYKERB or one of these other medicines. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
Each TYKERB tablet contains the active ingredient lapatinib ditosilate monohydrate, equivalent to 250 mg of lapatinib.
What TYKERB contains
| Active ingredient (main ingredient) | Lapatinib ditosilate monohydrate (lapatinib) |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | TYKERB tablets do not contain lactose, sucrose, tartrazine, or any other azo dyes. |
Do not take this medicine if you are allergic to any of these ingredients.
What TYKERB looks like
TYKERB is supplied in plastic bottles containing 70 tablets.
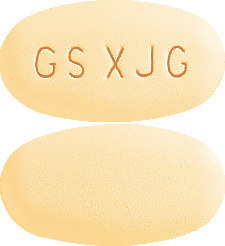
TYKERB 250 mg tablets are oval, rounded on both sides, yellow film-coated, and with GS XJG imprinted on one side (AUST R 185997).
Who distributes TYKERB
Tykerb is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
54 Waterloo Road,
Macquarie Park, NSW 2113, Australia
Telephone 1 800 671 203
ABN 18 004 244 160
www.novartis.com.au
® = Registered Trademark
This leaflet was prepared in November 2023.
Internal document code:
tyk270522c_v2 based on PI tyk270522i.
Published by MIMS December 2023

 No age based differences in the safety or efficacy of these regimens were observed. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Greater sensitivity of elderly individuals cannot be ruled out.
No age based differences in the safety or efficacy of these regimens were observed. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Greater sensitivity of elderly individuals cannot be ruled out.







 In the Tykerb plus letrozole treatment group, the most commonly observed study medication related serious adverse events were decreased left ventricular ejection fraction (LVEF) (2% of patients, compared to 1% for letrozole plus placebo) and diarrhoea (2% of patients, compared to < 1% for letrozole plus placebo). Other study medication related serious adverse events, including skin rash, hepatotoxicity and pneumonitis, were observed in < 1% of patients. The most common adverse events leading to discontinuation of treatment in the Tykerb plus letrozole treatment group were diarrhoea (4%) and vomiting (2%) (see Table 10).
In the Tykerb plus letrozole treatment group, the most commonly observed study medication related serious adverse events were decreased left ventricular ejection fraction (LVEF) (2% of patients, compared to 1% for letrozole plus placebo) and diarrhoea (2% of patients, compared to < 1% for letrozole plus placebo). Other study medication related serious adverse events, including skin rash, hepatotoxicity and pneumonitis, were observed in < 1% of patients. The most common adverse events leading to discontinuation of treatment in the Tykerb plus letrozole treatment group were diarrhoea (4%) and vomiting (2%) (see Table 10).
 The response rate (complete or partial response) independently assessed was 22% in the Tykerb plus capecitabine group compared with 14% in the capecitabine group (p = 0.091); similar results were observed for the clinical benefit response rate (complete response + partial response + stable disease for at least 6 months), which was 27% vs 18% (p = 0.069) in the combination versus the monotherapy arm, respectively.
The response rate (complete or partial response) independently assessed was 22% in the Tykerb plus capecitabine group compared with 14% in the capecitabine group (p = 0.091); similar results were observed for the clinical benefit response rate (complete response + partial response + stable disease for at least 6 months), which was 27% vs 18% (p = 0.069) in the combination versus the monotherapy arm, respectively. An independent data monitoring committee (IDMC) initially reviewed the results of the interim analysis (which included data from 321 of the 324 patients) and recommended that further enrollment into the study be halted due to a statistically significant and clinically relevant increase in TTP for the combination of Tykerb and capecitabine over capecitabine alone, which crossed a predefined statistical stopping boundary for superiority. At the time enrollment was halted (3 April, 2006), a total of 399 patients had been randomised to study treatment.
An independent data monitoring committee (IDMC) initially reviewed the results of the interim analysis (which included data from 321 of the 324 patients) and recommended that further enrollment into the study be halted due to a statistically significant and clinically relevant increase in TTP for the combination of Tykerb and capecitabine over capecitabine alone, which crossed a predefined statistical stopping boundary for superiority. At the time enrollment was halted (3 April, 2006), a total of 399 patients had been randomised to study treatment.







 In the intent to treat (ITT) population, investigator determined PFS was greater between the two treatment arms (see Table 19). Although statistically significant, the difference was not considered clinically relevant.
In the intent to treat (ITT) population, investigator determined PFS was greater between the two treatment arms (see Table 19). Although statistically significant, the difference was not considered clinically relevant.
