What is in this leaflet
This leaflet answers some common questions about VEDILOL tablets.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking VEDILOL against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What VEDILOL is used for
VEDILOL is used to treat heart failure.
VEDILOL belongs to a group of medicines called beta-blockers. These medicines work by relaxing tightened blood vessels and slowing the heart rate. VEDILOL has the additional effect of being an antioxidant.
Heart failure occurs when the heart can no longer pump blood strongly enough for the body's needs. Often the heart grows in size to try to improve the blood flow but this can make the heart failure worse.
Symptoms of heart failure include shortness of breath and swelling of the feet or legs due to fluid build-up.
VEDILOL reduces the pressure that the heart has to pump against as well as controlling your heart rate. Over 6 months or more this will reduce the size of an oversized heart and increase its efficiency.
VEDILOL reduces the chances of you being admitted to hospital and/ or dying from this condition.
VEDILOL is often used with other medicines to treat heart failure.
Your doctor, however, may have prescribed VEDILOL for another purpose.
Ask your doctor if you have any questions about why VEDILOL has been prescribed for you.
VEDILOL is not addictive.
This medicine is available only with a doctor's prescription.
Before you take VEDILOL
When you must not take it
Do not take VEDILOL if:
- you have had an allergic reaction to VEDILOL or any ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include an itchy skin rash, shortness of breath or swelling of the face or tongue.
- you have asthma or other conditions which make you short of breath from time to time.
- you have allergic disorders, including allergies which cause asthma or nose running/congestion.
- you have a history of a very slow heart rate or uneven heart beating.
- you have certain other heart conditions.
- you have liver problems including liver failure.
- you have very low blood pressure.
- the package is torn or shows signs of tampering.
- the expiry date (EXP) printed on the pack has passed or if the tablets appear damaged in some way.
If you take this medicine after the expiry date has passed, it may not work as well.
If you are not sure if you should be taking VEDILOL, talk to your doctor.
Do not give VEDILOL to people under 18 years of age. Safety and effectiveness in children has not been established.
Before you start to take it
Tell your doctor if:
- you are pregnant or plan to become pregnant. It is not known whether VEDILOL is harmful to an unborn baby when taken by a pregnant woman. If there is a need to take VEDILOL when you are pregnant, your doctor will discuss the risks and benefits to you and the unborn baby.
- you are breastfeeding or plan to breastfeed.VEDILOL passes into breast milk. Your doctor will discuss the risks and benefits of taking VEDILOL if you are breast-feeding.
- you have any other health problems, especially the following:
- angina or chest pain/tightness which occurs even when you are at rest (also called unstable angina)
- low blood pressure
- high blood pressure which varies widely
- very poor circulation to your fingers and/or toes (also called peripheral vascular disease)
- a history of poor kidney function
- chronic bronchitis or emphysema causing breathing difficulties
- diabetes
- sudden low blood sugar levels (also called hypoglycaemia)
- thyroid disorders
- severe allergic reactions causing swelling and/or difficulty breathing
- a rare cancer called phaeochromocytoma
- skin disease such as psoriasis (hardened patches of red skin)
- you are allergic to any other medicines, foods, dyes or preservatives.
- you plan to have surgery. Your surgeon and anaesthetist should know well ahead of the date of your surgery so they can allow for your condition and medications.
If you have not told your doctor about any of the above, tell them before you start taking VEDILOL.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you have bought from a pharmacy, supermarket or health food shop.
Some medicines may interfere with VEDILOL. These medicines include:
- rifampicin, a medicine used to treat tuberculosis (e.g. Rimycin®, Rifadin®)
- cimetidine, a medicine used to treat stomach ulcers or reflux (e.g. Tagamet®, Cimehexal®, Magicul®, Sigmetadine®)
- digoxin, a medicine used to treat heart failure (e.g. Lanoxin®)
- monoamine-oxidase inhibitors (MAOIs) such as phenelzine (Nardil®) and tranylcypromine (Parnate®), medicines used to treat depression
- clonidine, a medicine used to treat high blood pressure, migraine or menopausal symptoms (e.g. Catapres®)
- diltiazem, a medicine used to treat high blood pressure or angina (e.g. Cardizem®, Auscard®, Cardcal®, Coras®, Diltahexal®, Diltiamax®, Dilzem®, Vasocardol®)
- verapamil, a medicine used to treat high blood pressure, angina or fast heart rate (e.g. Isoptin®, Cordilox®, Anpec®, Verahexal®)
- drugs for when your heart doesn't beat smoothly, including disopyramide (Rythmodan®, Norpace®), quinidine (Kinidin®), procainamide (Pronestyl®), mexiletine (Mexitil®), lignocaine, flecainide (Tambocor®) and amiodarone (Cordarone®, Aratac®).
- drugs for diabetes, including insulin injections, glibenclamide (Daonil®, Glimel®), metformin (Diabex®, Diaformin®, Glucohexal®, Glucomet®, Glucophage®, NovoMet®), chlorpropamide (Diabinese®), gliclazide (Diamicron®), glipizide (Minidiab®, Melizide®) and tolbutamide (Rastinon®).
- cyclosporin, a medicine used to treat certain problems with the immune system.
These medicines may be affected by VEDILOL, or may affect how well it works. You may need to use different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking VEDILOL.
Ask your doctor or pharmacist if you are not sure about this list of medicines.
How to take VEDILOL
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
How much to take
Take VEDILOL exactly as your doctor has prescribed.
Your doctor will tell you how many VEDILOL tablets to take each day.
The usual starting dose in heart failure is 3.125mg twice daily. The dose is usually increased every two weeks to 6.25mg twice daily, 12.5mg twice daily and then 25mg twice daily. However, this may be done more slowly if side effects occur. If the tablets slow your heart too much you may go back to a lower dose.
Your doctor will monitor you carefully each time the dose is increased.
How to take it
Swallow tablets whole or halved with a glass of water. Do not crush or chew the tablets.
When to take it
Take VEDILOL during or immediately after a meal, at about the same times each day. If you take VEDILOL on an empty stomach, it may increase the risk of some of the side effects.
How long to take it
Your doctor will probably ask you to take VEDILOL long-term to control your heart failure.
Continue taking VEDILOL until your doctor tells you to stop. It is very important that VEDILOL is not ceased suddenly. If you are to stop taking VEDILOL, your doctor will advise you to reduce the dose slowly over approximately two weeks.
If you forget to take it
Do not take an extra dose. Wait until the next dose and take your normal dose then.
Do not try to make up for the dose that you missed by taking more than one dose at a time.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering your dose, ask your pharmacist for some hints.
In case of an overdose
Immediately telephone your doctor, or Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much VEDILOL. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
The following are some symptoms, which may or may not occur.
- low blood pressure, causing dizziness or fainting
- a very slow heart rate
- difficulty breathing
- vomiting
- shock
- seizures.
Keep telephone numbers for these places handy.
If you are not sure what to do, contact your doctor or pharmacist.
While you are taking VEDILOL
Things you must do
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Make sure you drink enough water during exercise and hot weather when you are taking VEDILOL, especially if you sweat a lot.
Tell all doctors, dentists and pharmacists who are treating you that you are taking VEDILOL. You should also tell your surgeon and anaesthetist if you are having surgery.
Tell your doctor if you become pregnant while taking VEDILOL.
Tell your doctor that you are taking VEDILOL if you are going to have any laboratory tests.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Tell your doctor if you feel the tablets are not helping your condition.
Be sure to keep all of your appointments with your doctor so that your progress can be checked. Your doctor may examine your eyes, and test your blood glucose and kidney function from time to time.
Things you must not do
Do not stop taking VEDILOL or change the dose without first checking with your doctor.
Do not let yourself run out of medicine over the weekend or on holidays. VEDILOL should only be stopped by gradually reducing the amount over a two-week period.
Do not give VEDILOL to anyone else even if they have the same condition as you.
Do not use VEDILOL to treat other complaints unless your doctor says to.
Do not take any other medicines whether they require a prescription or not without first telling your doctor or consulting a pharmacist.
Things to be careful of
Be careful driving or operating machinery until you know how VEDILOL affects you. VEDILOL may affect your ability to drive a car or operate machinery when started or when the dosage is increased.
If you wear contact lenses you may also notice a reduction in the amount of tear fluid in your eyes.
When taken with grapefruit juice the amount of VEDILOL absorbed by your body may be increased.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking VEDILOL. VEDILOL helps most people with heart failure but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- tiredness, drowsiness
- dizziness
- lightheadedness
- abnormal or blurry vision
- slow heart rate
- diarrhoea
- nausea or vomiting
- bronchitis
- high blood sugar
- weight increase
- fluid retention
These are the more common side effects of VEDILOL. Mostly these are mild and will decrease as you get used to your medicine.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- itching, dark urine, loss of appetite, yellowing of skin or eyes, or feeling "flu-like" with no clear cause.
- shortness of breath or swelling of the mouth or tongue
- uneven heart beating
- swelling of the feet or legs due to fluid build up
- bleeding or bruising more easily than normal
These may be serious side effects.
You may need urgent medical attention. Serious side effects are rare.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don't understand anything in this list.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After taking VEDILOL
Storage
Keep your tablets in the blister until it is time to take them.
Keep VEDILOL in a cool dry place where the temperature stays below 25 °C. Protect from moisture.
Do not store it, or any other medicine, in a bathroom or near a sink, or any other place where there is high humidity.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep VEDILOL where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking VEDILOL, or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
Availability
VEDILOL comes in 4 tablet strengths, 3.125mg, 6.25mg, 12.5mg and 25mg.
VEDILOL comes in blister pack sizes of:
- 3.125mg tablets - 30s
- 6.25mg tablets - 60s
- 12.5 mg tablets - 60s
- 25mg tablets - 60s
What it looks like
3.125mg: light pink, round biconvex uncoated tablet with “CL” scoreline “3” on one side.
6.25mg: yellow round biconvex uncoated tablet with “CL” scoreline “6” on one side.
12.5mg: orange round biconvex uncoated tablet with “CL” scoreline “12” on one side.
25mg: white to off-white round biconvex uncoated tablets with “CL” scoreline “25” on one side.
Ingredients
Active ingredient:
- VEDILOL 3.125, each tablet contains 3.125mg carvedilol
- VEDILOL 6.25, each tablet contains 6.25mg carvedilol
- VEDILOL 12.5, each tablet contains 12.5mg carvedilol
- VEDILOL 25, each tablet contains 25mg carvedilol
Inactive ingredients - each tablet also contains:
- sucrose
- lactose
- povidone
- crospovidone
- colloidal anhydrous silica
- magnesium stearate
- yellow iron oxide [172] (6.25mg and 12.5mg tablets only)
- red iron oxide [172] (3.125mg and 12.5mg tablets only).
VEDILOL tablets are gluten-free.
Sponsor
VEDILOL is supplied in Australia by:
Spirit Pharmaceuticals Pty Ltd
117 Harrington Street
The Rocks
Sydney, NSW 2000
Australia
Phone: 1800 065 772
Australian Registration Numbers:
Vedilol 3.125 Tablets
AUST R 136623
Vedilol 6.25 Tablets
AUST R 136873
Vedilol 12.5 Tablets
AUST R 137280
Vedilol 25 Tablets
AUST R 137281
This leaflet was prepared in November 2008
® Registered Trade Mark
Published by MIMS April 2009

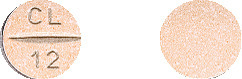





 The majority of patients were hospitalised for cardiovascular reasons. Treatment with carvedilol resulted in lower rates for almost all cardiac hospitalisations (worsening heart failure, atrial and ventricular and tachyarrhythmias, myocardial infarction and unstable angina pectoris).
The majority of patients were hospitalised for cardiovascular reasons. Treatment with carvedilol resulted in lower rates for almost all cardiac hospitalisations (worsening heart failure, atrial and ventricular and tachyarrhythmias, myocardial infarction and unstable angina pectoris). The following adverse events were reported more frequently with carvedilol in placebo controlled trials in patients with congestive heart failure.
The following adverse events were reported more frequently with carvedilol in placebo controlled trials in patients with congestive heart failure.