WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about VELPHORO.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet. You may need to read it again.
WHAT VELPHORO IS USED FOR
VELPHORO is used to control the phosphorus levels in the blood of patients with chronic kidney disease (CKD) on dialysis.
It contains 2.5 g of the active substance sucroferric oxyhydroxide which corresponds to 500 mg iron.
This medicine belongs to a group of drugs used when the levels of phosphorus in blood are too high (a condition called hyperphosphataemia).
The active ingredient works by binding phosphate from food in the digestive tract. This reduces how much is absorbed and the levels of phosphorus in the blood.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
BEFORE YOU TAKE VELPHORO
When you must not take it
Do not take this medicine if you have an allergy to:
- the active substance sucroferric oxyhydroxide, or to any of the other ingredient(s) listed at the end of this leaflet
- any other similar medicines
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you have too much iron in your body (haemochromatosis) or any other problems with the iron levels in your body.
Do not take this medicine after the expiry date printed on the pack or 90 days after opening (whichever is earlier) or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor or pharmacist before taking VELPHORO if you have:
- inflammation of the thin tissue (serous membrane) that lines the inner wall of the abdomen (peritonitis),
- significant stomach and/or liver disorders,
- had major surgery on your stomach and/or intestines,
- a history of abnormal iron accumulation in your bodily organs (haemochromatosis) or other iron disorders
- an intolerance to some sugars
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Your doctor will discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start taking VELPHORO.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and VELPHORO may interfere with each other. These include:
- alendronate (a medicine used to treat or prevent osteoporosis)
- doxycycline (antibiotics to treat infections)
- levothyroxine (a medicine used to treat thyroid problems)
These medicines may be affected by VELPHORO or may affect how well it works. You may need different amounts of your medicines. It is recommended that these drugs should be administrated at least 1 hour before or at least 2 hours after intake of Velphoro.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
HOW TO TAKE VELPHORO
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions, ask your doctor or pharmacist for help.
How much to take
The standard dose for this medicine is one tablet three times a day.
The usual recommended starting dose is 1,500 mg iron per day (1 tablet, 3 times a day).
The maximum recommended dose is 3,000 mg iron (2 tablets, 3 times a day).
Your doctor may adjust the dose during the treatment course depending on the phosphorus level in your blood.
Your doctor may have prescribed a different dose.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, VELPHORO may not work as well and your problem may not improve.
How to take it
VELPHORO chewable tablets should only be taken by mouth.
Take the tablet during a meal and chew it. DO NOT swallow it whole. If necessary, the tablet may be crushed to make this easier for you.
The amount of tablets taken per day should be divided across the three largest meals of the day.
No additional fluid above the amount usually taken due to your kidney disease is required.
Additional information for the blister pack:
- Separate the blister pack at perforations.
- Peel back the paper foil at the corner.
- Push the tablet through the aluminium foil.
When to take VELPHORO
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
Take your medicine during or immediately after a meal, at about the same time each day. If you take it on an empty stomach, it may cause stomach upset.
How long to take VELPHORO
Continue taking your medicine for as long as your doctor tells you. This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take it
Skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone Australia 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much VELPHORO. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
WHILE YOU ARE TAKING VELPHORO
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking VELPHORO.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor’s appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take VELPHORO to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor or pharmacist. If you stop taking it suddenly, your condition may worsen.
Things to be careful of
Be careful driving or operating machinery until you know how VELPHORO affects you. It is unlikely that this medicine will affect your ability to drive or to operate tools or machines. Ask your doctor if you are not sure.
SIDE EFFECTS
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking VELPHORO.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported in patients taking VELPHORO:
Very common (may affect more than 1 in 10 people): diarrhoea, discoloured faeces*.
Common (may affect up to 1 in 10 people): nausea, constipation, vomiting, indigestion, abdominal pain, flatulence, discolouration of teeth and/or tongue.
Uncommon (may affect up to 1 in 100 people): abdominal distension (bloating), gastritis, abdominal discomfort, difficulty swallowing, gastro-oesophageal reflux disorder (GORD), fatigue, itching skin, rash, headache, shortness of breath.
*As is often seen with oral iron preparations, discoloured (black) stools may occur very commonly in patients taking VELPHORO®. This may visually hide gastrointestinal bleeding. If you also have symptoms like increasing fatigue and breathlessness contact your doctor immediately.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may also occur in some people.
AFTER TAKING VELPHORO
Storage
Keep your medicine in the original container. If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 30°C.
Shelf life after first opening the bottle: 90 days
Do not store VELPHORO or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
VELPHORO chewable tablets are brown, circular, bi-planar tablets embossed with PA500 on one side.
The tablets are packed in high density polyethylene bottles with a child resistant polypropylene cap and a foil induction seal, or in child resistant aluminium blister packs.
VELPHORO is available in packs containing 30 or 90 chewable tablets.
Ingredients
Active ingredients:
- 2.5 g sucroferric oxyhydroxide (equivalent to 500 mg iron)
The active ingredient sucroferric oxyhydroxide contains 750 mg sucrose and 700 mg starches.
Inactive ingredients:
- woodberry flavour
- neohesperidin dihydrochalcone
- magnesium stearate
- silica (colloidal, anhydrous)
Sponsor
Vifor Pharma Pty Ltd
655 Elizabeth Street
Melbourne
VIC 3000, Australia
Tel: 1800 202 674
Australian Registration Number:
AUST R 216701
AUST R 216702
This leaflet was prepared in September 2023
Published by MIMS November 2023

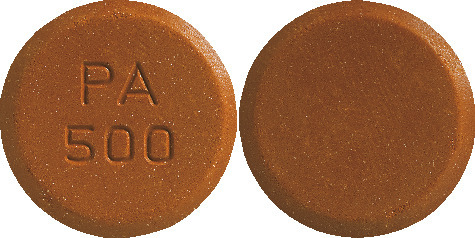
 Adverse drug reactions reported from the use of Velphoro at doses from 250 mg iron/day to 3000 mg iron/day in these patients (n = 835) are summarised in Table 2.
Adverse drug reactions reported from the use of Velphoro at doses from 250 mg iron/day to 3000 mg iron/day in these patients (n = 835) are summarised in Table 2.
 Mean serum phosphorus levels were 2.5 mmol/L at baseline and 1.8 mmol/L at week 12 for Velphoro (reduction by 0.7 mmol/L). Corresponding levels for sevelamer carbonate at baseline were 2.4 mmol/L and 1.7 mmol/L at week 12 (reduction by 0.7 mmol/L), respectively.
Mean serum phosphorus levels were 2.5 mmol/L at baseline and 1.8 mmol/L at week 12 for Velphoro (reduction by 0.7 mmol/L). Corresponding levels for sevelamer carbonate at baseline were 2.4 mmol/L and 1.7 mmol/L at week 12 (reduction by 0.7 mmol/L), respectively.
