SUMMARY CMI
XIFAXAN®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I taking XIFAXAN?
XIFAXAN contains the active ingredient rifaximin. XIFAXAN is used to help prevent a condition called hepatic encephalopathy (HE).
For more information, see Section 1. Why am I taking XIFAXAN? in the full CMI.
2. What should I know before I take XIFAXAN?
Do not take if you have ever had an allergic reaction to XIFAXAN or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take XIFAXAN? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with XIFAXAN and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take XIFAXAN?
- The recommended dosage of XIFAXAN is one tablet twice daily.
- Swallow the tablet whole with a glass of water. You can take XIFAXAN with or without food.
More instructions can be found in Section 4. How do I take XIFAXAN? in the full CMI.
5. What should I know while taking XIFAXAN?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking XIFAXAN? in the full CMI.
6. Are there any side effects?
Side effects that are common and less serious include swelling of the extremities, nausea, dizziness, fatigue, itching, muscle pain.
Serious side effects which need immediate medical attention include swelling of the face, lips or tongue which may make swallowing or breathing difficult, asthma, wheezing, shortness of breath, sudden or severe itching, skin rash, hives. If you suffer with cirrhosis (a type of liver disease), you may notice flu-like symptoms and painful rash or blistering affecting the skin, mouth, eyes, and genitals.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
XIFAXAN®
Active ingredient(s): Rifaximin
Consumer Medicine Information (CMI)
This leaflet provides important information about taking XIFAXAN. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about taking XIFAXAN.
Where to find information in this leaflet:
1. Why am I taking XIFAXAN?
2. What should I know before I take XIFAXAN?
3. What if I am taking other medicines?
4. How do I take XIFAXAN?
5. What should I know while taking XIFAXAN?
6. Are there any side effects?
7. Product details
1. Why am I taking XIFAXAN?
XIFAXAN contains the active ingredient rifaximin.
XIFAXAN is used to help prevent a condition called hepatic encephalopathy (HE).
Rifaximin is an antibiotic that passes through the gastrointestinal tract and very little is absorbed.
HE is a disease of the brain that occurs when the liver is not working properly. Symptoms are caused by too much ammonia in the blood. XIFAXAN works by killing bacteria in the gut that produce ammonia. This means less ammonia is produced and less gets into the blood.
XIFAXAN is intended to be used for preventing HE only in those patients where HE is likely to occur again, and where it cannot be managed with other treatments.
There is no experience using XIFAXAN to prevent the recurrence of HE in children or adolescents.
Ask your doctor or pharmacist if you have any questions about why XIFAXAN has been prescribed for you.
Your doctor may have recommended XIFAXAN for another reason.
2. What should I know before I take XIFAXAN?
Warnings
Do not take XIFAXAN if:
- You have an allergy to rifaximin or any of the rifamycin antibiotics (rifampicin, rifabutin) or to any other ingredient contained in this medicine, listed at the end of this leaflet. Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itchiness or hives on the skin.
- You have bowel obstruction (a blocked bowel).
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Check with your doctor if you:
- have any other medical conditions
- take any medicines for any other condition
- have allergies to any other medicines, foods, preservatives or dyes.
If you suffer with cirrhosis (a type of liver disease) you may experience severe skin reactions which may be life-threatening with painful rashes or blistering affecting the skin, mouth, eyes and genitals. If severe skin reactions or other allergic reactions occur, stop taking this medicine and seek urgent medical advice.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Your doctor can discuss with you the risks and benefits involved.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known if your baby can absorb rifaximin from breast milk if you are breastfeeding.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
There is a possibility that XIFAXAN can interact with other medicines, including ciclosporin, oral contraceptives and warfarin. Your doctor and pharmacist have more information on which medicines to be careful with or avoid while taking this medicine.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect XIFAXAN.
4. How do I take XIFAXAN?
How much to take
The recommended dosage of XIFAXAN is one tablet twice daily. Follow the instructions provided when XIFAXAN was prescribed.
When to take XIFAXAN
XIFAXAN should be taken orally twice daily.
How to take XIFAXAN
Swallow the tablet whole with a glass of water. You can take XIFAXAN with or without food.
If you forget to take XIFAXAN
XIFAXAN should be taken regularly at the same time each day. If you miss your dose at the usual time, take it as soon as you remember, and then go back to taking your medicine as you would normally.
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you take too much
If you think that you have taken too much XIFAXAN you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while taking XIFAXAN?
Things you should do
Call your doctor straight away if you:
If you develop watery and bloody diarrhoea (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of XIFAXAN you should see your doctor as soon as possible. If diarrhoea occurs, gets worse or does not improve during therapy, you should also contact your doctor.
Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care. Do not take any medicine to treat diarrhoea without first checking with your doctor.
Remind any doctor, dentist or pharmacist you visit that you are taking XIFAXAN.
Things you should not do
- Do not stop taking your medicine or lower the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
- Do not take XIFAXAN to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how XIFAXAN affects you.
Drinking alcohol
Tell your doctor if you drink alcohol.
As with all medicines, drinking alcohol while taking medicines is not recommended.
Looking after your medicine
- Keep your tablets in the blister packaging until it is time to take them. If you take the tablets out of the packaging they will not keep well.
- Keep your tablets in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Do not take this medicine after the expiry date.
The expiry date refers to the last day of that month.
Getting rid of any unwanted medicine
If you no longer need to take this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not take this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
If you suffer with cirrhosis (a type of liver disease) you may experience
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you have experienced, you can report side effects in Australia to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems and in New Zealand to the National Poisons Centre on 0800 764 766.
By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What XIFAXAN contains
| Active ingredient (main ingredient) | Rifaximin |
| Other ingredients (inactive ingredient) | Microcrystalline cellulose Glyceryl diisostearate Sodium starch glycolate Type A Colloidal anhydrous silica Purified talc The film coating contains Hypromellose Titanium dioxide Disodium edetate Propylene glycol Iron oxide red |
Do not take this medicine if you are allergic to any of these ingredients.
What XIFAXAN looks like
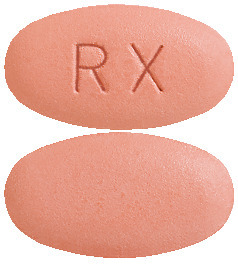
XIFAXAN are oval, biconvex, pink, film-coated tablets. (AUST R 183411).
Who distributes XIFAXAN
XIFAXAN is supplied in Australia by:
Norgine Pty Ltd
Suite 3.01 Building A
20 Rodborough Road
Frenchs Forest NSW 2086
AUSTRALIA
Phone: 1800 766 936
www.norgine.com.au
XIFAXAN is supplied in New Zealand by:
Pharmacy Retailing (NZ) Ltd
Trading as Healthcare Logistics
58 Richard Pearse Drive
Mangere, Auckland
NEW ZEALAND
(09) 918 5100
This leaflet was prepared in April 2023.
Published by MIMS June 2023

 The following adverse events, presented by body system, have also been reported in the placebo controlled clinical trial in greater than 2% but less than 5% of patients taking Xifaxan 550 mg orally two times a day for hepatic encephalopathy. The following includes adverse events occurring at a greater incidence than placebo (see Table 2).
The following adverse events, presented by body system, have also been reported in the placebo controlled clinical trial in greater than 2% but less than 5% of patients taking Xifaxan 550 mg orally two times a day for hepatic encephalopathy. The following includes adverse events occurring at a greater incidence than placebo (see Table 2).

 When the results were evaluated by the following demographic and baseline characteristics, the treatment effect of Xifaxan 550 mg in reducing the risk of breakthrough overt HE recurrence was consistent for: sex, baseline Conn score, duration of current remission and diabetes. The differences in treatment effect could not be assessed in the following subpopulations due to small sample size: non-white (n = 42), baseline MELD > 19 (n = 26), and those without concomitant lactulose use (n = 26).
When the results were evaluated by the following demographic and baseline characteristics, the treatment effect of Xifaxan 550 mg in reducing the risk of breakthrough overt HE recurrence was consistent for: sex, baseline Conn score, duration of current remission and diabetes. The differences in treatment effect could not be assessed in the following subpopulations due to small sample size: non-white (n = 42), baseline MELD > 19 (n = 26), and those without concomitant lactulose use (n = 26).

