What is in this leaflet
This leaflet answers some common questions about PROGOUT.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking PROGOUT against the benefits expected for you.or your child.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What PROGOUT is used for
PROGOUT is used in the treatment of:
- gouty arthritis or gout, a condition of painful swollen joints caused by uric acid crystals
- kidney stones
- other rare conditions where high levels of uric acid occur in the blood, for example Lesch-Nyhan syndrome.
PROGOUT helps to treat the symptoms of these conditions but will not cure them. It will not help treat the pain that occurs in an acute attack of gout.
PROGOUT belongs to a group of medicines called anti-uricaemic agents. These medicines reduce the amount of uric acid in the body. Most commonly, high levels of uric acid in the body are related to gout. Excess amounts of uric acid in the blood may lead to the development of crystals which deposit in the joints, causing pain, swelling and tenderness.
Ask your doctor if you have any questions about why PROGOUT has been prescribed for you. Your doctor may have prescribed PROGOUT for another reason.
PROGOUT is available only with a doctor's prescription.
There is no evidence that PROGOUT is addictive.
Use in children
There is not enough information to recommend the use of this medicine for children.
Before you take PROGOUT
When you must not take it
Do not take PROGOUT if you are allergic to medicines containing allopurinol (e.g. Zyloprim) or any of the ingredients listed at the end of this leaflet, including lactose monohydrate. Some of the symptoms of an allergic reaction may include skin rash, itching or hives; swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing; or other parts of the body; wheezing or difficulty breathing; shortness of breath; muscle pain or tenderness of joint pain.
Do not take PROGOUT if:
- you or a member of your immediate family have haemochromatosis, a condition where there is too much iron in the body, and you are also taking iron salts at the same time.
- you are having an acute attack of gout.
If a person first starts taking PROGOUT when they are having an attack of gout it can make the symptoms of this condition temporarily worse. However, if an acute attack of gout does occur when a person is already taking PROGOUT, it can be continued. Do not stop taking this medicine during an attack of gout unless advised by your doctor.
Do not take PROGOUT if the expiry date (EXP.) printed on the pack has passed. If you take this medicine after the expiry date, it may not work as well.
Do not take PROGOUT if the packaging shows signs of tampering or the tablets do not look like the tablets described at the end of this leaflet.
Before you start to take it
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will discuss the risks and benefits of taking PROGOUT during pregnancy.
Tell your doctor if you are breastfeeding or wish to breastfeed. PROGOUT passes into breast milk and may affect your baby. Your doctor will discuss the risks and benefits of taking PROGOUT when breastfeeding.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- liver problems
- kidney problems, including kidney stones
- high blood pressure
- heart failure or other heart problems
- diabetes mellitus
- epilepsy
- cancers or tumours
- conditions where the levels of uric acid are abnormally high
- haemochromatosis (a disease involving excessive deposits of iron in the body)
- thyroid problems.
Your doctor may want to take special care if you have any of these conditions.
Tell your doctor if you plan to have surgery.
If you have not told your doctor about any of the above, tell them before you start taking PROGOUT.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by PROGOUT, or may affect how well it works. These include:
- some medicines used to treat high blood pressure or heart problems
- aspirin and other salicylates
- probenecid, a medicine used to treat gout
- warfarin (Coumadin, Marevan), a medicine used to prevent blood clots
- azathioprine (e.g. Imuran) and ciclosporin (Neoral, Sandimmun), medicines used to prevent organ transplant rejection or to treat certain immune system problems
- mercaptopurine monohydrate (Puri-Nethol), a medicine used in the treatment of leukaemia
- chlorpropamide, a medicine for diabetes
- phenytoin (Dilantin), a medicine for epilepsy
- theophylline (Nuelin), a medicine used in asthma
- antibiotics called amoxicillin (e.g. Amoxil, Moxacin) and ampicillin (Alphacin)
- thiazide diuretics or fluid tablets (e.g.Dithiazide).
- aluminium hydroxide, a medicine used to treat the symptoms of too much stomach acid
- adenine arabinoside ('Vidarabine'), an anti-viral medicine
- medicines used for cancer (e.g. cyclophosphamide, doxorubicin)
- didanosine, used to treat HIV infection.
The above medicines may reduce the effectiveness of PROGOUT, reduce its own effectiveness and/or react with PROGOUT resulting in untoward or sometimes dangerous side effects.
The above list is not exhaustive.Your doctor can tell you what to do if you are taking any of these medicines.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking PROGOUT.
How to take PROGOUT
Follow all directions given to you by your doctor and pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle, ask your doctor or pharmacist.
How much to take
The dose varies from person to person. Your doctor will tell you how much to take.
The usual dose is 100 mg to 600 mg daily in divided doses (that is one 100mg tablet daily up to one 300mg tablet twice daily) but the dose may be as much as 900mg daily to treat very high blood levels of uric acid.
Elderly people over 65 years of age and those with kidney and/or liver problems usually receive the lowest dose possible to control uric acid production.
Children under 15 years of age usually take 100 mg to 400 mg daily in divided doses.
Your doctor may advise you to take a different dose. This depends on your condition and whether or not you are taking any other medicines.
How to take PROGOUT
Swallow the tablets with plenty of water to reduce the possibility of gastric upset.
When to take PROGOUT
Take PROGOUT during or immediately after food at the frequency directed by your doctor.
Take your medicine at the same time each day.
PROGOUT is usually taken once a day. However, if your dose is higher than 300 mg a day, your doctor may advise you to take it morning and night.
If you forget to take PROGOUT
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have any questions about this, check with your doctor or pharmacist.
How long to take PROGOUT for
To properly control your condition, PROGOUT must be taken every day.
PROGOUT will not cure your condition but will help control pain, stiffness and other symptoms.
Keep taking PROGOUT for as long as your doctor recommends.
If you take too much PROGOUT (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much PROGOUT. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
If you take too much PROGOUT, you may feel dizzy, nauseous, and experience vomiting and diarrhoea.
While you are taking PROGOUT
Things you must do
Drink at least 2 litres (8 to 10 glasses) of fluid each day. This will help to reduce the levels of uric acid in your body and prevent the formation of kidney stones.
Stop taking PROGOUT immediately if you develop a skin rash or any other sign of an allergic reaction. Before starting any new medicine, tell your doctor or pharmacist that you are taking PROGOUT.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking PROGOUT.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
If you become pregnant while taking PROGOUT, tell your doctor immediately.
Visit your doctor regularly so they can check on your progress. You may need to have blood or urine tests.
Tell your doctor if you feel PROGOUT is not helping your condition. If you continue to have painful attacks of gout your doctor may need to adjust your treatment.
Tell your doctor if, for any reason, you have not taken PROGOUT exactly as prescribed. Otherwise, your doctor may adjust your treatment unnecessarily.
Things you must not do
Do not take PROGOUT to treat an acute attack of gout. Your doctor will prescribe another medicine such as colchicine or a non-steroidal anti-inflammatory drug (NSAID) to relieve an acute attack of gout.
Do not use PROGOUT to treat any other conditions unless your doctor tells you to.
Do not give PROGOUT to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how PROGOUT affects you. PROGOUT may cause drowsiness, dizziness or lack of co-ordination in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Avoid drinking alcohol while taking PROGOUT. Combining PROGOUT and alcohol can make you more sleepy, dizzy or lightheaded. Alcohol may also increase the formation of uric acid.
Certain foods are best avoided when you have gout. Food such as organ meats, anchovies and yeast extracts (includes Vegemite®) can increase the levels of uric acid in your body. Ask your doctor or pharmacist for more advice about which foods to avoid.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking PROGOUT.
PROGOUT helps most people with gouty arthritis and kidney stones, but it may have unwanted side effects in some people.
Side effects only occur rarely in people taking PROGOUT. Most of the time they are minor. You may need medical treatment if you get some of the side effects.
People with liver and kidney problems have an increased chance of experiencing side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
The most common side effect is skin rash. Stop treatment with PROGOUT immediately and contact your doctor if a rash does occur.
Tell your doctor if you notice any of the following and they worry you:
- nausea, vomiting, diarrhoea
- headache
- oedema (swelling)
- drowsiness, dizziness, unsteadiness when walking
- high blood pressure
- abdominal pain
- skin rash
- blurred vision, vision problems
- confusion
- unexplained nosebleeds
- change in bowel habits
- change in taste sensation
- sleeplessness
- change in hair colour
If any of the following happen, stop taking PROGOUT and tell your doctor immediately, or go to Accident and Emergency at the nearest hospital:
- fatty stools
- going to the toilet often
- blood in the urine
- burning feeling while passing urine
- change in the amount of urine
- yellowing of the skin and eyes (jaundice)
- bleeding or bruising more easily
- swelling of the hands, ankles or legs
- hair loss
- general malaise or depression
- sleepiness
- confusion or vision problems
- numbness in the limbs
- angina (chest pain involving the heart)
- severe palpitations
- severe itching, skin flaking, skin rash or hives
- swelling of the face, lips or tongue which may cause difficulty in swallowing or breathing
- asthma, wheezing or shortness of breath
- pain or tightness in the chest
- if chills, fever, joint pain or swollen glands occur, especially if they occur together with or shortly after a skin rash.
- fainting, seizures or fits
These are very serious yet rare side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some patients. Some of these side effects (e.g. high blood pressure) can only be found when your doctor does tests from time to time to check your progress.
Check with your doctor as soon as possible if you have any problems while taking PROGOUT, even if you do not think the problems are connected with the medicine or are not listed in this leaflet.
After taking PROGOUT
Storage
Keep PROGOUT where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 25C.
Do not store PROGOUT or any other medicine in the bathroom or near a sink.
Do not leave PROGOUT in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking PROGOUT, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
PROGOUT tablets are available 2 strengths:
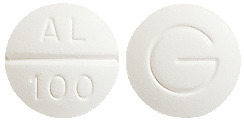
- PROGOUT 100 - 9.5mm white normal convex, tablet marked "AL/100" on one side and "G" on the other
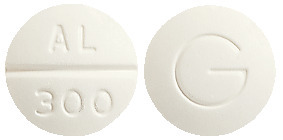
- PROGOUT 300 - 11mm white normal convex tablet marked "AL/300 " on one side and "G" on the other.
PROGOUT 100 is available in bottles of 200 tablets. PROGOUT 300 is available in bottles of 60 tablets.
Ingredients
The active ingredient in PROGOUT is allopurinol.
PROGOUT 100
Each PROGOUT 100 tablet contains 100 mg of allopurinol.
The tablets also contain:
- Lactose monohydrate
- maize starch
- povidone
- macrogol 8000
- sodium lauryl sulfate
- purified talc
- magnesium stearate.
PROGOUT 100 tablet contains galactose, sulfites and sugars (as lactose).
PROGOUT 300
Each tablet PROGOUT 300 contains 300 mg of allopurinol.
The tablets also contain:
- maize starch
- povidone
- maltodextrin
- sodium starch glycollate
- microcrystalline cellulose
- magnesium stearate.
PROGOUT 300 tablet contains sulfites.
Manufacturer
PROGOUT is made in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30 - 34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
PROGOUT 100 - AUST R 27969
PROGOUT 300 - AUST R 17708
This leaflet was prepared in May 2023.
PROGOUT® is a Viatris company trade mark
PROGOUT_cmi\May23/00
Published by MIMS June 2023

 Allopurinol decreases urate formation in two ways:
Allopurinol decreases urate formation in two ways: Molecular formula: C5H4N4O. Molecular weight: 136.1.
Molecular formula: C5H4N4O. Molecular weight: 136.1.