What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
The name of your medicine is APO-Aciclovir. It contains the active ingredient aciclovir.
The 200mg strength is used to:
- treat genital herpes. It makes an outbreak of genital herpes shorter and less severe
- prevent or reduce the number of outbreaks and/or severity of genital herpes in people who experience them often.
The 800mg strength is used:
- to treat shingles, also known as herpes zoster. Shingles is caused by the same virus which causes chicken pox. It usually involves nerve pain and a blistery rash, limited to one area of the body. If taken within 72 hours of first getting the rash, aciclovir makes an outbreak of shingles shorter and less severe
- as part of the management program for certain infections in people who have the human immunodeficiency virus (HIV). HIV is the virus that causes acquired immune deficiency syndrome (AIDS). Aciclovir does not cure AIDS or get rid of the HIV virus from your body, but it may prevent further damage to the immune system by stopping production of the herpes viruses.
It belongs to a group of medicines called anti-virals.
This medicine works by stopping the production of the virus that causes herpes and shingles.
Aciclovir does not get rid of the virus from your body.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
This medicine is not expected to affect your ability to drive a car or operate machinery.
This medicine should not be used in children.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- any medicine containing aciclovir or valaciclovir
- any of the ingredients listed at the end of this leaflet.
- any other similar medicines.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
- fainting or hay fever-like symptoms.
If you think you are having an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney or liver problems
- neurological disorders such as muscle weakness, paralysis, seizures, confusion, etc
- an imbalance of electrolytes (salts) in your body
- severe lack of oxygen from any part of your body
- neurological reactions from a cytotoxic (anti-cancer) medicine.
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if:
- You are planning to have surgery or an anaesthetic.
- You are currently receiving or are planning to receive dental treatment.
If you have not told your doctor about any of the above, tell him/ her before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and this one may interfere with each other. These include:
- probenecid, a medicine commonly used to treat gout
- cimetidine, used for stomach problems
- diuretics, also called fluid tablets
- interferon, used to treat multiple sclerosis, hepatitis, leukaemia, Hodgkin's lymphoma and other diseases
- methotrexate given by injection into the spine to treat cancer and leukaemia
- mycophenolate mofetil, used by people with organ transplants.
These medicines may be affected by this medicine or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Other medicines not listed above may also interact with acyclovir.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
The doses below may be lower if you have problems with your kidneys.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
Initial genital herpes
The usual dose is one 200mg tablet every four hours, while awake, for a total of five tablets daily for ten days.
Recurrent genital herpes
The usual does is one 200mg tablet three times a day for up to six months.
Or
One 200mg tablet every four hours, while awake, for a total of five tablets daily for five days.
Shingles
The usual dose is one 800mg tablet every four hours, while awake, for a total of five tablets daily for seven days (or up to ten days if your eyes are affected by shingles).
Management of HIV
The usual dose is one 800mg tablet four times a day at six hourly intervals.
How to take it
Swallow the tablets whole with a full glass of water.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much of this medicine, you may feel or be sick, have a headache and/or feel confused.
While you are using this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may occasionally do tests on your blood or urine to check for side effects and see how your kidneys are working. Go to your doctor regularly for a check-up.
Things you must not do
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Genital herpes and HIV can be transmitted to your partner during sexual activity. It is important to remember that this medicine will not keep you from transmitting herpes or HIV to others.
Be careful driving or operating machinery until you know how this medicine affects you.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine.
This medicine may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach problems such as nausea (feeling sick), vomiting (being sick), diarrhoea, constipation, stomach pain
- changes in taste sensation, loss of appetite, weight loss
- dizziness/giddiness or headache
- difficulty sleeping
- increased hair loss
- weakness, fatigue, lack of energy, tiredness
- aching, leg pains, muscles pains, joint pain, muscle cramps
- menstrual problems.
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following:
- confusion
- depression, agitation, irritability
- unusual thoughts or actions, hallucinations (seeing, feeling or hearing things that are not there)
- shakiness/trembling
- difficulty speaking
- uncoordinated movements, i.e. unsteady walking
- fever, sore throat, swollen glands
- blood problems (e.g. feeling tired and weak, fever, frequent infections, unusual bruising or bleeding or swelling around wounds)
- fluid retention
- eye problems (inflamed eye).
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- yellowing of the skin and/or eyes (jaundice) or other liver problems with a collection of symptoms which may include: mental confusion, drowsiness, restlessness, itching and unconsciousness
- kidney problems e.g. too much or too little urine, or pain when urinating, or pain in the kidneys
- troubled breathing
- chest pain, fast heart beat (palpitations)
- convulsion (fits)
- losing consciousness or in a coma
- signs of a blood clot such as a swollen and painful area in your leg, and swelling in your foot or ankle.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Storage and Presentation
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
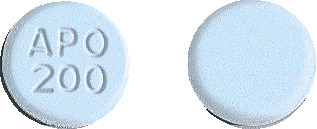
APO-Aciclovir 200mg tablets are round, blue, flat-faced, bevel-edged tablets, engraved "APO" over "200" on one side and the other side plain.
Blister packs of 25, 50 and 90 tablets.*
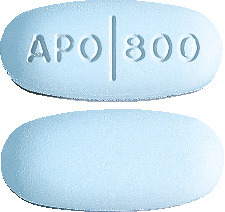
APO-Aciclovir 800mg tablets are oval, blue, biconvex tablet. Engraved APO partial bisect 800 on one side, plain on the other side. Blister packs of 35 tablets.
* Not all strengths, pack types and/or pack sizes may be available
APO-Aciclovir 200mg tablets:
AUST R 159140.
APO-Aciclovir 800mg tablets:
AUST R 159141.
Ingredients
This medicine contains 200 mg or 800 mg of aciclovir as the active ingredient.
This medicine also contains the following:
- lactose (for the 200mg strength only)
- magnesium stearate
- colloidal anhydrous silica
- microcrystalline cellulose
- indigo carmine
- brilliant blue FCF (for the 800mg strength only).
The 200mg tablets does not contain gluten, sucrose, tartrazine or any other azo dyes.
The 200mg tablets contains sugars as lactose.
The 800mg tablets does not contain any lactose, gluten, tartrazine or any other azo dyes.
Manufacturer/Distributor/ Supplier
This medicine is made/distributed/ supplied in Australia by:
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
APO and APOTEX are registered trademarks of Apotex Inc.
This leaflet was prepared in June 2018
Published by MIMS August 2018