What is in this leaflet
This leaflet answers some common questions about MELIZIDE.
It does not contain all of the available information. It does not take the place of talking to your doctor, pharmacist or diabetes educator.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking MELIZIDE against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor, pharmacist or diabetes educator.
Keep this leaflet with your medicine. You may need to read it again.
What MELIZIDE is used for
MELIZIDE is used to control blood sugar in patients with Type 2 diabetes mellitus. This type of diabetes is also known as non-insulin dependent diabetes mellitus (NIDDM) or maturity onset diabetes.
MELIZIDE is used when diet and exercise are not enough to control your blood sugar (glucose). It can be used alone, or together with insulin or other medicines for treating diabetes.
MELIZIDE is available only with a doctor's prescription.
There is no evidence that MELIZIDE is addictive.
How MELIZIDE works
MELIZIDE belongs to a group of medicines called sulfonylureas. These medicines lower high blood glucose by increasing the amount of insulin produced by your pancreas.
If your blood glucose is not properly controlled, you may experience hypoglycaemia (low blood glucose) or hyperglycaemia (high blood glucose).
Hypoglycaemia (low blood glucose) can occur suddenly. Signs may include:
- weakness, trembling or shaking
- sweating
- lightheadedness, dizziness, headache or lack of concentration
- irritability
- tearfulness or crying
- hunger
- numbness around the lips and tongue.
If not treated quickly, these may progress to:
- loss of co-ordination
- slurred speech
- confusion
- fits or loss of consciousness.
Hyperglycaemia (high blood glucose) usually occurs more slowly than hypoglycaemia.
Signs of hyperglycaemia may include:
- lethargy or tiredness
- headache
- thirst
- passing large amounts of urine
- blurred vision.
Hyperglycaemia can lead to serious problems with your heart, eyes, circulation or kidneys.
Ask your doctor if you have any questions about why MELIZIDE has been prescribed for you.
You doctor may have prescribed it for another reason.
Use in Children
There is not enough evidence to recommend the use of MELIZIDE in children.
Before you take MELIZIDE
When you must not take it
Do not take MELIZIDE if you are allergic to:
- any medicine containing glipizide
- other sulphonylureas
- sulphur antibiotics (e.g. sulphonamides)
- certain types of fluid tablets (thiazide diuretics)
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take MELIZIDE if you have any of the following medical conditions:
- Type 1 diabetes mellitus, also known as insulin dependent diabetes mellitus
- severe or unstable diabetes
- diabetic ketoacidosis with or without coma
- gangrene
- severe thyroid disease
- severe kidney disease
- severe liver disease
- infection or high temperature
- severe trauma
- major surgery.
Do not take MELIZIDE if you are pregnant or plan to become pregnant. Insulin is more suitable for controlling blood glucose during pregnancy. Your doctor will replace MELIZIDE with insulin while you are pregnant.
Do not take MELIZIDE if you are breastfeeding or plan to breastfeed. It is not known whether MELIZIDE passes into breast-milk. There could be a possibility that your baby is affected.
Do not give MELIZIDE to children. Safety and effectiveness in children have not been established.
Do not take MELIZIDE after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking MELIZIDE, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, dyes or preservatives.
Tell your doctor if you have or have had any of the following medical conditions:
- adrenal or pituitary problems
- glucose-6-phosphate dehydrogenase (G6PD) deficiency, a condition in which the body does not have enough of the enzyme G6PD
- liver problems
- kidney problems.
Tell your doctor if:
- you ever drink alcohol
- you do not eat regular meals
- you do a lot of exercise
- you are feeling ill or unwell.
Alcohol, diet, exercise and your general health all strongly affect the control of your diabetes.
If you have not told your doctor about any of the above, tell him/her before you start taking MELIZIDE.
Taking other medicines
Tell your doctor, pharmacist or diabetes educator if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop. Some medicines and MELIZIDE may interfere with each other.
a. Some medicines may lead to low blood glucose (hypoglycaemia) by increasing the blood-glucose-lowering effect of MELIZIDE. These include:
- alcohol
- some medicines used to treat high blood pressure and other heart conditions (beta-blockers, ACE inhibitors, diazoxide)
- some medicines used to treat arthritis, pain and inflammation (salicylates e.g. aspirin; non-steroidal anti-inflammatory drugs)
- some antibiotics (sulphonamides, chloramphenicol)
- medicines used to prevent blood clots (coumarin derivatives)
- medicines used to treat fungal infections (miconazole, fluconazole)
- some cholesterol-lowering medicines (clofibrate)
- other medicines used to treat diabetes (biguanides)
- probenecid (a medicine used to treat gout or to increase the blood levels of some antibiotics)
- some medicines used to treat depression (monoamine oxidase inhibitors)
- some medicines used to treat reflux and ulcers (H2 receptor antagonists, e.g. cimetidine)
- some medicines used to treat cancer (cyclophosphamide).
b. Some medicines may lead to a loss of control of your diabetes by weakening the blood glucose-lowering effect of MELIZIDE. These include:
- alcohol
- some medicines used to treat high blood pressure (calcium channel blocking medicines)
- glucagon, a medicine used to treat low blood glucose
- corticosteroids such as prednisone and cortisone
- some medicines used to treat tuberculosis (isoniazid)
- nicotinic acid (used for the lowering of blood fats)
- oestrogens, progestogens, oral contraceptives and certain other hormonal treatments such as danazol. These medicines are used in birth control, Hormone Replacement Therapy (HRT), or to treat other women's health problems
- some medicines used to treat mental illness or psychotic disorders (phenothiazines)
- phenytoin, a medicine used to treat epilepsy (convulsions)
- diuretics also known as fluid tablets (thiazides)
- some asthma medicines, preparations for coughs and colds, and weight-reducing medicines (sympathomimetics)
- thyroid hormones
- some medicines used to treat cancer (cyclophosphamide).
c. MELIZIDE may change the effect of some other medicines. These include:
- barbiturates (used for sedation).
Tetracycline, a type of antibiotic, can interfere with the measurement of glucose in the urine.
You may need different amounts of your medicine or you may need to take different medicines.
Your doctor, pharmacist or diabetes educator can tell you what to do if you are taking any of these medicines. They also have more information on medicines to be careful with or avoid while taking MELIZIDE.
How to take MELIZIDE
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
The dose varies from patient to patient. Your doctor will recommend how many tablets to take each day.
The usual starting dose is 1 tablet taken before breakfast. However, a lower starting dose may be needed in older people or those with liver problems.
Your doctor may increase or decrease the dose depending on your blood glucose levels.
How to take it
Swallow the tablets whole with a full glass of water.
When to take it
Take MELIZIDE about half an hour before meals. Meal times are generally when your blood glucose levels are highest. Taking MELIZIDE half an hour before a meal helps your body produce insulin when it is needed most.
Do not skip meals while taking MELIZIDE.
How long to take it for
Continue taking MELIZIDE for as long as your doctor tells you. MELIZIDE helps to control diabetes but does not cure it. Therefore, you may have to take it for a long time.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Missed doses can cause hyperglycaemia.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much MELIZIDE (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much MELIZIDE. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much MELIZIDE, you may experience symptoms of hypoglycaemia (low blood glucose).
If not treated quickly, these symptoms may progress to loss of co-ordination, slurred speech, confusion, fits or loss of consciousness.
At the first signs of hypoglycaemia, raise your blood glucose quickly by eating jelly beans, sugar or honey, drinking a non-diet soft drink, or taking glucose tablets.
While you are taking MELIZIDE
Things you must do
If you are about to be started on any new medicine, tell your doctor or pharmacist that you are taking MELIZIDE.
Tell any other doctors, dentists and pharmacists who are treating you that you are taking MELIZIDE.
If you become pregnant while taking MELIZIDE, tell your doctor immediately.
Make sure that you, your friends, family and work colleagues can recognise the symptoms of hypoglycaemia and hyperglycaemia and know how to treat them.
If you experience any of the symptoms of hypoglycaemia (low blood glucose), you need to raise your blood glucose immediately. You can do this by doing one of the following:
- eating 5-7 jelly beans
- eating 3 teaspoons of sugar or honey
- drinking half a can of non-diet soft drink
- taking 2-3 concentrated glucose tablets.
Unless you are within 10 to 15 minutes of your next meal or snack, follow up with extra carbohydrates such as plain biscuits, fruit or milk. Taking this extra carbohydrate will prevent a second drop in your blood glucose level.
If you are elderly or are taking other medicines for diabetes such as insulin or metformin, the risk of hypoglycaemia is increased.
If you experience any of the symptoms of hyperglycaemia (high blood glucose), contact your doctor immediately.
The risk of hyperglycaemia is increased in the following situations:
- uncontrolled diabetes
- illness, infection or stress
- taking less MELIZIDE than prescribed
- taking certain other medicines
- too little exercise
- eating more carbohydrates than normal.
Tell your doctor if you become ill, or experience extra stress, injury, fever, injection or need surgery. Your blood glucose may become difficult to control at these times. Your doctor may replace MELIZIDE with insulin.
Make sure you check your blood glucose levels regularly. This is the best way to tell if your diabetes is being controlled properly. Your doctor or diabetes educator will show you how and when to do this.
Keep all of your doctor's appointments so that your progress can be checked.
Carefully follow your doctor's and dietician's advice on diet, drinking alcohol and exercise.
Tell your doctor immediately if you notice the return of any symptoms you had before starting MELIZIDE. These may include lethargy or tiredness, headache, thirst, passing large amounts of urine and blurred vision. These may be signs that MELIZIDE is no longer working, even though you may have been taking it successfully for some time.
Things you must not do
Do not take MELIZIDE to treat any other complaints unless your doctor tells you to.
Do not give MELIZIDE to anyone else, even if they have the same condition as you.
Do not stop taking MELIZIDE, or change the dose, without checking with your doctor.
Do not skip meals while taking MELIZIDE.
Things to be careful of
Be careful driving or operating machinery until you know how MELIZIDE affects you. MELIZIDE may cause dizziness and drowsiness in some people. If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
Be careful not to let your blood glucose levels fall too low. Low blood glucose levels may slow your reaction time and affect your ability to drive or operate machinery.
Protect your skin when you are in the sun, especially between 10 am and 3 pm. If outdoors, wear protective clothing and use a minimum of 30+ sunscreen. If your skin does appear to be burning, tell your doctor immediately. MELIZIDE may cause your skin to be more sensitive to sunlight than it is normally. Exposure to sunlight may cause skin rash, itching, redness or severe sunburn.
Be careful when drinking alcohol while you are taking this medicine. If you drink alcohol while taking MELIZIDE, you may get flushing, headache, breathing difficulties, rapid heart beat, stomach pains or feel sick and vomit.
Things that would be helpful for your condition
Some self help measures suggested below may help your condition. Your doctor or pharmacist can give you more information about these measures.
If you are travelling it is a good idea to:
- wear some form of identification showing you have diabetes
- carry some form of sugar to treat hypoglycaemia if it occurs e.g. jelly beans, sugar sachets
- carry emergency food rations in case of delay e.g. dried fruit, biscuits
- keep MELIZIDE readily available
Sick days
If you become sick with a cold, fever or flu, it is very important to continue taking MELIZIDE, even if you feel unable to eat your normal meal. If you have trouble eating solid food, use sugar-sweetened drinks as a carbohydrate substitute, or eat small amounts of bland food. Your diabetes educator or dietician can give you a list of foods to use for sick days.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking MELIZIDE. MELIZIDE helps most people with diabetes, but it may have unwanted side effects in some people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- signs of hypoglycaemia which may include weakness, trembling or shaking, sweating, lightheadedness, headache, dizziness, sleepiness, irritability, tearfulness or crying, hunger and lack of concentration
- confusion, shaking and feeling generally unwell. These may be experienced but are usually mild and transient. However, they may also be symptoms of hypoglycaemia.
- stomach upset including nausea (feeling sick), vomiting and stomach cramps or pain
- diarrhoea, constipation
- dizziness, drowsiness, headache. These may be experienced but are usually mild and transient. However, they may also be symptoms of hypoglycaemia.
- rashes, sores, redness, itching, or eczema. Sometimes these effects may disappear following continued treatment but you should ask your doctor for advice if you experience skin problems while taking MELIZIDE.
- unusual weight gain
- visual disturbances which may include blurred vision, double vision and abnormal vision. These may be experienced but are usually mild and transient. However, they may also be symptoms of hypoglycaemia.
- symptoms of sunburn such as redness, itching or blistering which may occur more quickly than normal
Tell your doctor as soon as possible if you notice any of the following:
- yellowing of the eyes or skin (jaundice)
- bleeding or bruising more easily than normal, reddish or purplish blotches under the skin
- signs of frequent infections, such as fever, chills, sore throat or mouth ulcers
Tell your doctor immediately or go to Accident and Emergency at the nearest hospital if you notice any of the following:
- signs of anaemia such as tiredness, being short of breath, looking pale and seizure.
- signs of liver disease such as nausea, vomiting, loss of appetite, feeling generally unwell, fever, itching, yellowing of the skin or eyes, and dark coloured urine.
The above list includes serious side effects you may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
This is not a complete list of all possible side effects. Other side effects not listed above may also occur in some people.
After using MELIZIDE
Storage
Keep MELIZIDE where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in the blister pack until it is time to take them. The blister packaging will help protect the tablets.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store MELIZIDE or any other medicine in the bathroom or near a sink.
Do not leave MELIZIDE in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If your doctor tells you to stop taking MELIZIDE, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
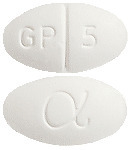
MELIZIDE is an oval, white tablet marked GP|5 and a Greek Alpha symbol.
Each pack contains 100 tablets.
Ingredients
The active ingredient in MELIZIDE is glipizide. Each MELIZIDE tablet contains 5 mg of glipizide.
The tablets also contain:
- lactose monohydrate
- starch - maize
- starch - pregelatinised maize
- magnesium stearate.
This medicine does not contain gluten. This medicine contains trace amounts of galactose and sulfites.
Manufacturer
MELIZIDE is made in Australia by:
Alphapharm Pty Ltd
Level 1, 30 The Bond
30 - 34 Hickson Road
Millers Point NSW 2000
www.mylan.com.au
Australian registration number:
AUST R 46946
This leaflet was prepared on 7 July 2020.
MELIZIDE_cmi\Jul20/00
Published by MIMS September 2020

 Chemical name: 1-cyclohexyl- 3-{4-[2-(5-methylpyrazine- 2-carboxamido) ethyl] benzene-sulfonyl} urea.
Chemical name: 1-cyclohexyl- 3-{4-[2-(5-methylpyrazine- 2-carboxamido) ethyl] benzene-sulfonyl} urea.