What is in this leaflet
This leaflet answers some common questions about alendronate. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What this medicine is used for
Alendronate is used to treat osteoporosis. It belongs to a group of medicines called bisphosphonates.
Osteoporosis is a disease which causes bones to become more porous, gradually making them weaker, more brittle and likely to break.
Understanding Bone
Bone is living, growing tissue. Throughout life, our bodies are breaking down old bone and rebuilding new bone in a continuous cycle.
How it works
Alendronate works by slowing down the process of old bone being removed, which allows the bone-forming cells time to rebuild normal bone. Alendronate helps prevent the loss of bone and helps to rebuild bone, making bone less likely to break. This helps to prevent or reverse the progression of osteoporosis.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed alendronate for another reason.
This medicine is available only with a doctor's prescription.
This medicine is not addictive.
There is not enough information to recommend the use of alendronate in children.
Before you take this medicine
Alendronate can irritate or burn your mouth or food pipe (also called the oesophagus). The chances of this happening should be reduced if you follow the instructions for taking alendronate in this leaflet.
When you must not take it
Do not take this medicine if you have an allergy to:
- alendronate
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue, throat or other parts of the body
- itching or hives on the skin
Do not take this medicine if you have the following medical conditions:
- certain disorders of the food pipe (oesophagus)
- conditions that cause difficulty in swallowing
- unable to stand or sit upright for at least 30 minutes
- low blood calcium (hypocalcaemia)
Do not take this medicine if you are pregnant, plan to become pregnant or are breastfeeding. Alendronate has not been studied in pregnant or breast-feeding women.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- serious kidney disease
- swallowing or digestive problems, such as stomach pain, reflux, ulcers, Barrett's oesophagus
- low vitamin D levels
- diabetes
- alcohol abuse
- any bone fractures
Tell your doctor if you have any of the following, which may increase your risk of getting jaw bone problems:
- cancer
- radiotherapy, chemotherapy treatment
- taking angiogenesis inhibitors
- problems with your teeth, mouth or gums
- anaemia
- problems with blood clotting
- infection
- you are a smoker
- you are taking steroids
- you are planning to have any dental procedures such as tooth extraction or other oral surgery
- you don't regularly look after your teeth or see a dentist.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with alendronate if taken at the same time. These include:
- antacids
- calcium supplements
- non-steroidal anti-inflammatory drugs (NSAIDs), e.g. ibuprofen.
Take alendronate at least 30 minutes before taking any of these or other oral medicines.
Other medicines not listed above may also interact with alendronate.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. They may differ to the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
The usual dose is one 70 mg tablet once a week.
How to take it
Swallow one tablet whole with a full glass of plain water only. It is important to take alendronate with plain water only, not mineral water. Mineral water and other drinks, including fruit juices, coffee and tea, will reduce the effect of alendronate by interfering with its absorption into the body.
Do not crush, chew or suck on a tablet of alendronate. Mouth ulcers or irritation to your mouth or food pipe (oesophagus) may occur if the tablet is crushed, or chewed or dissolved in the mouth.
Stay upright for at least 30 minutes after swallowing the tablet and do not take any food, medicines or drinks other than plain tap water during this time.
Do not lie down immediately after swallowing it. It is important to stay upright (sitting, standing or walking around) for at least 30 minutes after swallowing your tablet. It is also very important to stay upright until after you have eaten your first food of the day. These actions will help make sure the tablet reaches your stomach quickly and help reduce the potential for irritation to your food pipe (oesophagus).
When to take it
Choose a day of the week that best fits your schedule. Every week take one tablet on your chosen day.
Take alendronate immediately after getting up for the day. Do not take it at bedtime, or before you get up in the morning.
Alendronate is effective only if taken when your stomach is empty. Food, drinks other than plain water, and other medicines will lessen the effect of alendronate by interfering with its absorption into the body.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
Alendronate can only prevent or treat your osteoporosis, by helping prevent further loss of bone and continuing to rebuild bone, if you take it every week.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If you miss a tablet, take one tablet on the morning after you remember.
Do not take two tablets on the same day. This may increase the chance of you getting an unwanted side effect.
Return to taking one tablet once a week, as originally scheduled on your chosen day.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too many tablets at one time, drink a full glass of milk or take some antacids. Do not make yourself vomit and do not lie down. If you take too much alendronate, you may have an upset stomach, heartburn, throat or stomach pain or problems swallowing.
While you are taking this medicine
Things you must do
If you develop difficulty or pain upon swallowing, chest pain, or new or worsening heartburn, stop taking the tablets and call your doctor.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell your doctor immediately if you develop pain in your hip or thigh. This may be a sign that you have a stress fracture of your hip or upper thigh bone.
Tell your doctor immediately if you break a bone, or you develop bone, muscle or joint pain.
Tell your doctor and dentist if you require a dental procedure, or if you develop toothache, jaw pain, delayed healing or infection.
Tell your doctor if you are going to have surgery.
Keep up good oral hygiene practices by:
- regularly brushing teeth
- cleaning between teeth with floss or interdental cleaner
- eating a balanced diet
- visiting your dentist regularly
Make sure you receive enough calcium and Vitamin D in your diet.
Your doctor, dietician or pharmacist can tell you what foods you should eat, and whether you should take a supplement.
Things you must not do
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not take your medicine to treat any other complaint unless your doctor tells you to.
Do not stop taking your medicine, or change the dosage, without first checking with your doctor.
Things that would be helpful for your osteoporosis
Talk to your doctor or pharmacist about the following self-help measures for more information:
- Exercise can help to build and maintain strong bones.
- Eat a balanced diet, ensuring you have enough calcium and Vitamin D in your diet.
- Smoking may increase the rate at which you lose bone, increasing your risk of fracture. You may need to stop or cut down on smoking.
- Drinking alcohol excessively on a regular basis may increase your risk of developing osteoporosis. You may need to cut down on the amount of alcohol you drink.
Things to be careful of
Be careful driving or operating machinery until you know how this medicine affects you. Alendronate can make some people feel giddy or dizzy or have blurred vision.
You may take aspirin while you are being treated with alendronate. However, both aspirin and alendronate may increase the chance of stomach upsets.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking alendronate.
This medicine helps most people with osteoporosis, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- stomach complaints, such as pain, wind, an uncomfortable feeling in the stomach, belching after eating, heartburn, nausea, vomiting
- unusual taste
- hair loss
- constipation, diarrhoea
- headache
- aching muscles, joints or bones
- flu-like symptoms, such as aching muscles, generally feeling unwell, and rarely, fever
- swollen joints
- dizziness or spinning sensation
- unusual tiredness or weakness
- dry skin
- swelling of the hands, ankles or feet
The above list includes the more common side effects. Mostly, these are mild.
Tell your doctor as soon as possible if you notice any of the following:
- jaw-bone problems, infection, delayed healing after teeth are pulled out or other work that involves drilling into the jaw (tell both your doctor and dentist immediately)
- skin rash or redness of the skin, sometimes made worse by sunlight; itchiness
- mouth ulcers
- blurred vision, pain or redness in the eye
- muscle cramps or spasms or tingling sensation in the fingers or around the mouth (signs of low blood calcium levels)
- difficulty or pain when swallowing; painful mouth; chest pain; new or worsening heartburn (may be due to irritation or ulceration of the mouth or food pipe. Stop taking the tablets and tell your doctor immediately).
The above list includes serious side effects that may need medical attention. Most of these side effects are rare.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to Accident and Emergency at your nearest hospital:
- shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, or other parts of the body; rash, itching or hives on the skin; severe skin reactions (e.g. severe rash and/or peeling layers of skin) (signs of an allergic reaction)
- black tar-like and/or bloody stools (may indicate severe stomach or duodenal ulcers)
- pain in your hip or thigh bone (signs of stress fractures of the upper thigh or hip)
The above list includes very serious side effects and are usually very rare. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may occur in some patients.
Storage and disposal
Storage
Keep your medicine in its pack until it is time to take it. If you take your medicine out of the pack it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine left over.
Product description
What it looks like
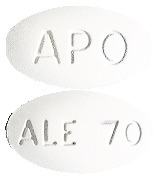
White, oval biconvex tablet, engraved APO on one side and ALE 70 on the other side.
Contained in blister packs of 4 tablets. AUST R 123863.
Ingredients
Each tablet contains alendronate sodium trihydrate equivalent to 70 mg alendronic acid as the active ingredient.
It also contains the following:
- magnesium stearate
- mannitol
- microcrystalline cellulose
This medicine does not contain gluten, lactose, sucrose, tartrazine or any other azo dyes.
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel St,
Cremorne VIC 3121
Web: www.arrotex.com.au
Arrotex Pharmaceuticals Pty Ltd is the licensee of the registered trademarks APO and APOTEX from the registered proprietor Apotex Inc.
This leaflet was prepared in March 2023.
Published by MIMS May 2023

 Rarely, rash and erythema have occurred.
Rarely, rash and erythema have occurred.
 These increases were highly significant relative both to baseline and placebo at each measurement site in each study. Increases in BMD were evident as early as three months and continued throughout the entire three years of treatment. See Figure 2 for lumbar spine results. In the two year extension of these studies, treatment with alendronate 10 mg/day resulted in continued increases in BMD at the lumbar spine and trochanter (absolute additional increases between years 3 and 5: lumbar spine 0.94%; trochanter 0.88%). BMD at the femoral neck, forearm and total body were maintained. Thus, alendronate appears to reverse the progression of osteoporosis as assessed by increased bone mineral density. Alendronate was similarly effective regardless of age, race, baseline rate of bone turnover, renal function and use of concomitant medications.
These increases were highly significant relative both to baseline and placebo at each measurement site in each study. Increases in BMD were evident as early as three months and continued throughout the entire three years of treatment. See Figure 2 for lumbar spine results. In the two year extension of these studies, treatment with alendronate 10 mg/day resulted in continued increases in BMD at the lumbar spine and trochanter (absolute additional increases between years 3 and 5: lumbar spine 0.94%; trochanter 0.88%). BMD at the femoral neck, forearm and total body were maintained. Thus, alendronate appears to reverse the progression of osteoporosis as assessed by increased bone mineral density. Alendronate was similarly effective regardless of age, race, baseline rate of bone turnover, renal function and use of concomitant medications. In patients with post-menopausal osteoporosis treated with alendronate 10 mg/day for one or two years the effects of treatment withdrawal were assessed. Following discontinuation, there were no further increases in bone mass and the rates of bone loss were similar to those in the placebo groups. These data indicate that continuous treatment with alendronate is required to produce progressive increases in bone mass.
In patients with post-menopausal osteoporosis treated with alendronate 10 mg/day for one or two years the effects of treatment withdrawal were assessed. Following discontinuation, there were no further increases in bone mass and the rates of bone loss were similar to those in the placebo groups. These data indicate that continuous treatment with alendronate is required to produce progressive increases in bone mass. Furthermore, in this population of patients with baseline vertebral fracture, treatment with alendronate significantly reduced the incidence of hospitalisations resulting from any cause (25.0% versus 30.7%, a 20% relative risk reduction). This difference appears to be related, at least in part, to the reduction in fracture incidence.
Furthermore, in this population of patients with baseline vertebral fracture, treatment with alendronate significantly reduced the incidence of hospitalisations resulting from any cause (25.0% versus 30.7%, a 20% relative risk reduction). This difference appears to be related, at least in part, to the reduction in fracture incidence.
 Preclinical studies show that any drug that is not deposited in bone is rapidly excreted in the urine. No evidence of saturation of bone uptake was found over three weeks in rats with a cumulative intravenous dose of 35 mg/kg. Although no clinical information is available, it is likely that, as in animals, elimination of alendronate via the kidney will be reduced in patients with impaired renal function. Therefore, somewhat greater accumulation of alendronate in bone might be expected in patients with impaired renal function (see Section 4.2 Dose and Method of Administration).
Preclinical studies show that any drug that is not deposited in bone is rapidly excreted in the urine. No evidence of saturation of bone uptake was found over three weeks in rats with a cumulative intravenous dose of 35 mg/kg. Although no clinical information is available, it is likely that, as in animals, elimination of alendronate via the kidney will be reduced in patients with impaired renal function. Therefore, somewhat greater accumulation of alendronate in bone might be expected in patients with impaired renal function (see Section 4.2 Dose and Method of Administration). Chemical name: (4-amino-1-hydroxybutylidene) bisphosphonic acid monosodium salt trihydrate.
Chemical name: (4-amino-1-hydroxybutylidene) bisphosphonic acid monosodium salt trihydrate.