SUMMARY CMI
APO-Azathioprine
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using APO-Azathioprine?
APO-Azathioprine contains the active ingredient azathioprine. APO-Azathioprine is used to help prevent the body from rejecting transplanted organs such as the heart or kidney.
For more information, see Section 1. Why am I using APO-Azathioprine? in the full CMI.
2. What should I know before I use APO-Azathioprine?
Do not use it if you have ever had an allergic reaction to azathioprine or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use APO-Azathioprine? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with APO-Azathioprine and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use APO-Azathioprine?
- Take his medicine at about the same time each day. Taking at the same time each day will have the best effect. It will also help you remember when to take it.
- Take this medicine at least one hour before or three hours after food or milk.
More instructions can be found in Section 4. How do I use APO-Azathioprine? in the full CMI.
5. What should I know while using APO-Azathioprine?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using APO-Azathioprine? in the full CMI.
6. Are there any side effects?
The common side effects include any infection or fever, unexpected bruising or bleeding, black stools or blood in the urine or stools, new marks on skin or any change to marks that may have been there previously, headache, stiff neck and extreme sensitivity to bright light, nausea and vomiting, tiredness, dizziness or generally unwell, irregular heartbeat, sores in the mouth and on the lips, change in sense of smell or taste.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
APO-Azathioprine
Active ingredient(s): Azathioprine
Consumer Medicine Information (CMI)
This leaflet provides important information about using APO-Azathioprine. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using APO-Azathioprine.
Where to find information in this leaflet:
1. Why am I using APO-Azathioprine?
2. What should I know before I use APO-Azathioprine?
3. What if I am taking other medicines?
4. How do I use APO-Azathioprine?
5. What should I know while using APO-Azathioprine?
6. Are there any side effects?
7. Product details
1. Why am I using APO-Azathioprine?
APO-Azathioprine contains the active ingredient Azathioprine. APO-Azathioprine belongs to a group of medicines called immunosuppressants. It works by suppressing the body's immune defence system.
Azathioprine is usually taken in combination with other medicines such as corticosteroids or other immunosuppressants.
APO-Azathioprine is used to help prevent the body from rejecting transplanted organs such as the heart or kidney.
It is also used to treat autoimmune diseases, where your immune system is reacting against your own body. These include:
- Severe rheumatoid arthritis
- Systemic lupus erythematous
- Chronic active hepatitis
- Certain skin muscle, and blood disease.
2. What should I know before I use APO-Azathioprine?
Warnings
Do not use APO-Azathioprine if:
- you are allergic to Azathioprine, or any of the ingredients listed at the end of this leaflet.
- Always check the ingredients to make sure you can use this medicine.
- Do not take this medicine if you have an allergy to:
- Azathioprine
- 6-mercaptopurine
- Any of the ingredients listed at the end of this leaflet. - Some of the symptoms of an allergic reaction may include:
- Shortness of breath
- Wheezing or difficulty breathing
- Swelling of the face, lips, tongue, throat, or other parts of the body.
- Rash, itching or hives on the skin
Check with your doctor if you:
- have any other medical conditions
- liver or kidney disease
- a condition where your body produces too little of a natural chemical called thiopurine methyltransferase (TPMT)
- Lesch-Nyhan Syndrome
- Chicken pox or shingles
- Hepatitis B
- Irritable bowel syndrome
- A history of cytomegalovirus disease - take any medicines for any other condition
- Do not take this medicine if you have rheumatoid arthritis previously treated with alkylating agents (medicines such as chlorambucil, melphalan or cyclophosphamide).
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Azathioprine may cause birth defects if either male or female is taking it at the time of conception. Both you and your partner should take adequate contraceptive precautions while taking Azathioprine to prevent pregnancy.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Azathioprine is not recommended for use during breastfeeding as it passes into breastmilk and may affect your baby.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins, or supplements that you buy without a prescription from your pharmacy, supermarket, or health food shop.
Before you start to take it
- Tell your doctor if you have allergies to any other medicines, foods, preservatives, or dyes.
- Tell your doctor if you have recently been vaccinated or immunized or plan to get a vaccination or immunization. Azathioprine may affect the way the vaccine work or your reaction to the vaccine.
- Tell your doctor if you are planning to have surgery or an anesthetic.
- Tell your doctor if you are currently receiving or are planning to receive dental treatment. Tell your dentist that you are taking Azathioprine Dental work, whenever possible, should be completed before you start taking Azathioprine or delayed until your blood cell counts are normal.
Taking other medicines
Some medicines and azathioprine may interfere with each other. These include:
- Pencillamine, used in the treatment of rheumatoid arthritis.
- Captopril, used in high blood pressure and heart failure
- Cimetidine, used in stomach ulcers and ingredients
- Indomethacin, used as a painkiller
- Co-trimoxazole, ketoconazole, erythromycin and rifampicin used to treat infections
- Allopurinol, oxipurinol, thiopurinol, or febuxostat, used mainly to treat gout
- Tubocurarine and succinylcholine used during anesthesia.
- Frusemide, may be used to reduce swelling caused by excess fluid
- Warfarin, used to prevent blood clots
- Mesalazine, olsalazine or sulphasalazine, used in the treatment of ulcerative colitis
- Phenytoin, phenobarbital, rifampicin, ketoconazole or erythromycin
- Methotrexate, used in the treatment of cancer
- Ribavirin, used to treat a type of respiratory infection
- Infliximab, used to treat autoimmune diseases
These medicines may be affected by azathioprine or may affect how well it works. You may need different amount of medicine, or you may need to take different medicines.
Your doctor and pharmacists have more information on medicines to be careful with or avoid while taking this medicine.
Other medicines not listed above may also interact with azathioprine.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect APO-Azathioprine.
4. How do I use APO-Azathioprine?
How much to take / use
- Follow all directions given to you by your doctor or pharmacist carefully.
- They may differ to the information contained in this leaflet.
- If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
- Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicine.
When to take / use APO-Azathioprine
- APO-Azathioprine should be used at about the same time each day.
- Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
- Take this medicine at least one hour before or three hours after food or milk.
- Food can interfere with the absorption of this medicine.
If you forget to use APO-Azathioprine
APO-Azathioprine should be used regularly at the same time each day.
If it's almost time to take your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses.
This may increase the chance of you experiencing side effects.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you use too much APO-Azathioprine
If you think that you have used too much APO-Azathioprine, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
If you use too much azathioprine you may get an unexpected infection, ulcers in the throat, bruising and bleeding.
5. What should I know while using APO-Azathioprine?
Things you should do
Call your doctor straight away if you:
- If you are about to be started on any new medicine, remind your doctor or pharmacist that you are taking this medicine.
- If you become pregnant, father a child, or start to breastfeed while taking this medicine, tell your doctor immediately.
- Tell your doctor if you plan to have any vaccinations or immunisations. This medicine may affect the way some vaccines work, or your reaction to the vaccine.
- Tell your doctor if you are about to have any blood tests.
- Tell your doctor if you are going to have surgery or an anesthetic or going into hospital.
- Keep all your doctors' appointments so that your progress can be checked.
Your doctor will perform blood tests every week for the first eight weeks, then at least once a month after that, while you are taking azathioprine.
Before you start azathioprine, your doctor should also test for thiopurine methyltransferase (TPMT) enzyme deficiency. - Try to avoid contact with people who have infectious diseases, such as the flu, chickenpox, or shingles.
Tell your doctor immediately if you do come into contact with someone who has chickenpox or shingles. - Avoid contact with sports or other situations where bruising or injury may occur. Be careful to avoid cutting yourself with sharp objects (e.g., razors)
- Protect yourself from the sun while you are taking azathioprine.
If you go out in the sun, wear a hat, protective clothing, and use sunscreen. - Tell your doctor immediately if you notice new moles, changes in existing moles, lumps on your body or you feel unwell.
Azathioprine suppresses your immune system.
Lowering your body's immune defence system increases your risk of skin cancer, cervical cancer, lymphoma, and other cancers. - If you are a female, tell your doctor if you notice unusual vaginal discharge or bleeding, and make sure to have regular Pap smears.
Remind any doctor, dentist, or pharmacist you visit that you are using APO-Azathioprine.
Things you should not do
- Do not give this medicine to anyone else even if they have the same condition as you.
- Do not take your medicine to treat any other complaints unless your doctor tells you to.
- Do not stop taking your medicine or change the dosage without first checking with your doctor.
If you stop it suddenly, your condition may worsen
Things to be careful of
Driving or using machines
Be careful before you drive or use any machines or tools until you know how APO-Azathioprine affects you.
APO-Azathioprine may cause dizziness and tiredness in some people. If you have any of these symptoms, do not drive or operate machinery or do anything else that is dangerous.
Drinking alcohol
Tell your doctor if you drink alcohol.
Alcohol may have some effects while taken with APO-Azathioprine.
Looking after your medicine
- Keep your medicine in the pack until it is time to take it.
If you take the medicine out of the pack It may not keep well. - Keep your medicine in a cool and dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat, or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on windowsills.
Keep it where young children cannot reach it.
A locked cupboard at least one-and-a-half-meters above the ground is a good place to store medicines.
When to discard your medicine
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your doctor what to do with any medicine that is left over.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Very Serious side effects
| Very Serious side effects | What to do |
Side-effects reported particularly in organ transplanted patients are:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these very serious side effects. You may need urgent hospitalization. |
Other side effects not listed above may occur in some patients.
Some of these side effects can only be found when your doctor does tests from time to time to check your progress (e.g., low blood cell count)
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What APO-Azathioprine contains
| Active ingredient (Main ingredient) | Azathioprine |
| Other ingredients (Inactive ingredients) | Lactose monohydrate Microcrystalline cellulose Maize starch Magnesium stearate Purified water Opadry complete film coating system 03B52231 yellow (25mg tablet only) Opdary clear YS-1R-7006 (50mg tablet only) |
| Potential allergens | N/A |
This medicine contains sugars as lactose.
This medicine does not contain gluten, sucrose, tartrazine, and other azo dyes.
Do not take this medicine if you are allergic to any of these ingredients.
What APO-Azathioprine looks like
25mg tablets
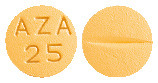
APO-Azathioprine is orange coloured, round, biconvex, film coated tablet with “AZA 25” embossed on one side and break line on other side. (Aust R 205759).
50mg tablets
APO-Azathioprine is pale yellow, coloured, round, biconvex film coated tablet with “AZA 50” embossed on one side and break line on other side. AUST R 205762
Available in packs of 100 tablets.
*Not all strengths, pack types and/or pack sizes may be available.
Who distributes APO-Azathioprine
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Australia
Web: www.arrotex.com.au
This leaflet was prepared in November 2023.
Published by MIMS January 2024


 Chemical name: 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7-H-purine.
Chemical name: 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7-H-purine.