SUMMARY CMI
AVSARTAN HCT Tablets
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about taking this medicine, speak to your doctor or pharmacist.
1. Why am I taking AVSARTAN HCT?
AVSARTAN HCT contains the active ingredients irbesartan and hydrochlorothiazide. AVSARTAN HCT is used to treat high blood pressure, which doctors call hypertension. For more information, see Section 1. Why am I taking AVSARTAN HCT? in the full CMI.
2. What should I know before I take AVSARTAN HCT?
Do not take if you have ever had an allergic reaction to AVSARTAN HCT or any of the ingredients listed at the end of the CMI. Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I take AVSARTAN HCT? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with AVSARTAN HCT and affect how it works. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take AVSARTAN HCT?
- Your doctor will tell you how many tablets to take each day. The usual dose is one tablet per day.
- Swallow the table whole with a glass of water and take AVSARTAN HCT at about the same time each day.
More instructions can be found in Section 4. How do I take AVSARTAN HCT? in the full CMI.
5. What should I know while taking AVSARTAN HCT?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking AVSARTAN HCT? in the full CMI.
6. Are there any side effects?
Common side effects: headache; dizziness or light-headedness (vertigo), unusual tiredness or fatigue, pain in the stomach or gut nausea and/or vomiting, sexual problems. Serious side effects: swelling of the face, lips, tongue or throat which may cause difficulty in swallowing or breathing; severe and sudden onset of pinkish, itchy swellings on the skin (hives or nettle rash). Refer to the CMI for the full list. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
AVSARTAN HCT Tablets
Active ingredient(s): irbesartan and hydrochlorothiazide
Consumer Medicine Information (CMI)
This leaflet provides important information about taking AVSARTAN HCT. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about taking AVSARTAN HCT.
Where to find information in this leaflet:
1. Why am I taking AVSARTAN HCT?
2. What should I know before I take AVSARTAN HCT?
3. What if I am taking other medicines?
4. How do I take AVSARTAN HCT?
5. What should I know while taking AVSARTAN HCT?
6. Are there any side effects?
7. Product details
1. Why am I taking AVSARTAN HCT?
AVSARTAN HCT contains the active ingredients irbesartan and hydrochlorothiazide. Both medicines reduce blood pressure in different ways.
Irbesartan belongs to a group of medicines known as angiotensin-II receptor antagonists. Angiotensin II is a substance produced in the body that causes blood vessels to narrow. Irbesartan blocks angiotensin-II and therefore widens your blood vessels, making it easier for your heart to pump blood throughout your body. This helps to lower your blood pressure.
Hydrochlorothiazide belongs to the class of medicines known as diuretics. Diuretics cause an increase in the volume of urine. They also help with lowering blood pressure particularly when combined with other blood pressure reducing medicines.
AVSARTAN HCT is used to treat high blood pressure, which doctors call hypertension.
2. What should I know before I take AVSARTAN HCT
Warnings
Do not take AVSARTAN HCT if:
- you are allergic to irbesartan, hydrochlorothiazide or to sulfonamide derived medicines (commonly known as sulfur drugs), or to any ingredients listed at the end of this leaflet. Always check the ingredients to make sure you can take this medicine.
- you are pregnant (or think you may be pregnant) or are planning to become pregnant.
- you are breast-feeding
- you have diabetes or have kidney problems and you are being treated with medicines that lower blood pressure such as an ACE inhibitor, any aliskiren-containing medicines that belong to a group of medicines known as AIIRAs (medicines also used to treat high blood pressure)
- you are not producing urine
Check with your doctor if you:
- have any other medical conditions
- have had recent excessive vomiting or diarrhea or think you are dehydrated
- have kidney problems, or have had a kidney transplant or dialysis
- have heart problems
- have liver problems, or have had liver problems in the past
- have diabetes
- have gout or have had gout in the past
- have lupus erythematosus
- have high or low levels of potassium or sodium or other electrolytes in your blood
- have primary aldosteronism. This is a condition which causes sodium retention and an increase in blood pressure.
- are strictly restricting your salt intake
- have had a sympathectomy. This is a surgical procedure to cut or block a nerve in your body.
- have been taking diuretics. These are medicines that reduce fluid retention in your body and are often used to treat high blood pressure.
- have had skin cancer or you develop an unexpected skin lesion during the treatment. Treatment with hydrochlorothiazide, particularly long term use with high doses, may increase the risk of some types of skin and lip cancer (non-melanoma skin cancer). Protect your skin from sun exposure and UV rays while taking AVSARTAN HCT.
- take any medicines for any other condition
- are pregnant or intend to become pregnant
- if you are breast-feeding or plan to breastfeed
- plan to have surgery (even at the dentist) that needs a general anaesthetic
- have a history of allergy or asthma
- have allergies to any substances, such as foods, preservatives or dyes.
- have a decrease in your vision or pain in one or both of your eyes. If so, you should discontinue AVSARTAN HCT tablet treatment and seek prompt medical attention, as these could be symptoms of fluid accumulation in the eye (choroidal effusion) or an increase of pressure in your eye (glaucoma) and can happen within hours to weeks of taking AVSARTAN HCT tablets. This can lead to permanent vision loss, if not treated. If you have had a penicillin or sulfonamide (sulfur drug) allergy, you can be at higher risk of developing this.
- experienced breathing or lung problems (including inflammation or fluid in the lungs) following hydrochlorothiazide intake in the past.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Use in children
AVSARTAN HCT should not be given to children.
Pregnancy and breastfeeding
- Check with your doctor if you are pregnant or intend to become pregnant. Do not take AVSARTAN HCT if you are pregnant or intend to become pregnant.
- Talk to your doctor if you are breastfeeding or intend to breastfeed. Do not take AVSARTAN HCT if you are breastfeeding.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor if you are taking or plan to take any of the following:
- other medicines to treat high blood pressure
- other diuretics
- lithium or lithium-containing medicines (for example lithium carbonate)
- potassium tablets
- potassium-containing salt tablets
- non-steroidal anti-inflammatory medicine such as diclofenac, ibuprofen) and COX-2 inhibitors (for example celecoxib) These medicines may be used to relieve pain, swelling and other symptoms of inflammation including arthritis. Taking AVSARTAN HCT and an anti-inflammatory medicine alone or with a thiazide diuretic (fluid tablet) may damage your kidneys. It may also reduce the effect AVSARTAN HCT has on lowering blood pressure.
- alcoholic drinks
- sleeping tablets
- strong pain medicines such as codeine or morphine
- medicines to treat diabetes, such as repaglinide. AVSARTAN HCT might induce hypoglycaemia; low blood sugar.
- calcium supplements or medicines containing calcium
- medicines containing Vitamin D
- medicines for gout
- powder or granules used to help reduce cholesterol (cholestyramine or colestipol hydrochloride)
- heart medicines such as digoxin or medicines to treat abnormal heart rhythm such as sotalol hydrochloride
- corticosteroid medicines such as prednisone, cortisone or adrenocorticotropic hormone (ACTH) medicines used to treat cancer
- amantadine, a medicine used to treat Parkinson's disease or to prevent influenza
- anticholinergic medicines, these can be used to treat Parkinson's disease, to relieve stomach cramps or spasms or used to prevent travel sickness
- carbamazepine, a medicine used to treat convulsion
- medicines used during surgery
- medicines used in an emergency such as adrenaline
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect AVSARTAN HCT.
4. How do I take AVSARTAN HCT?
How much to take
- AVSARTAN HCT will usually be prescribed by your doctor if previous treatment does not produce a sufficient drop in your blood pressure. Your doctor will tell you how to switch from your previous treatment to AVSARTAN HCT
- The usual dose of AVSARTAN HCT is one tablet once a day.
The full blood pressure lowering effect should be reached 6 to 8 weeks after beginning treatment. - If your blood pressure is not satisfactorily reduced with AVSARTAN HCT, your doctor may prescribe another medicine to be taken with AVSARTAN HCT
- Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
- Follow the instructions provided and take AVSARTAN HCT until your doctor tells you to stop.
When to take AVSARTAN HCT
- Take AVSARTAN HCT at about the same time each day.
How to take AVSARTAN HCT
- Swallow the tablet whole with a glass of water. It does not matter whether you take AVSARTAN HCT tablets before or after food.
- Continue taking AVSARTAN HCT until your doctor tells you to stop.
- To help you remember to take your tablets each day, AVSARTAN HCT tablets are supplied in a Calendar pack with the foil backing marked with the days of the week. This is just a way to help you to remember to take your tablets. All of the tablets in the pack are the same.
- When you start a new strip of tablets, take the tablet marked "START" at the end of the blister strip. On the next day, take the tablet marked with the relevant day of the week. Continue taking your tablets each day until all of the tablets are taken. Commence the next strip at "START" and continue as before.
If you forget to take AVSARTAN HCT
AVSARTAN HCT should be used regularly at the same time each day.
If you miss your dose at the usual time and if it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you take too much AVSARTAN HCT
If you think that you have used too much AVSARTAN HCT, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre(
by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
If you take too many AVSARTAN HCT tablets you will probably feel light-headed or dizzy.
5. What should I know while taking AVSARTAN HCT
Things you should do
- Tell any other doctors, dentists, and pharmacists who are treating you that you are taking AVSARTAN HCT
- Tell your doctor immediately if you become pregnant while taking AVSARTAN HCT
- Have your blood pressure checked when your doctor tells you to, to make sure AVSARTAN HCT is working
- If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking AVSARTAN HCT
- If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking AVSARTAN HCT. Your blood pressure may drop suddenly
- Make sure you drink enough water during exercise and hot weather when you are taking AVSARTAN HCT, especially if you sweat a lot. If you do not drink enough water while taking AVSARTAN HCT, you may faint or feel light-headed or sick. This is because your body does not have enough fluid and your blood pressure is low. If you continue to feel unwell, tell your doctor.
- Tell your doctor if you have excessive vomiting and/or diarrhoea while taking AVSARTAN HCT. You may lose too much water and salt and your blood pressure may drop too much.
- Tell your doctor immediately if you feel lightheaded or dizzy after taking your first dose of AVSARTAN HCT, or when your dose is increased.
- Tell your doctor if you experience an increased sensitivity of the skin to the sun with symptoms of sunburn (such as redness, itching, swelling, blistering) occurring more quickly than normal.
- If you develop any shortness of breath or difficulty breathing after taking AVSARTAN HCT, seek medical attention immediately.
Things you should not do
- Do not give AVSARTAN HCT tablets to anyone else, even if they have the same condition as you
- Do not take AVSARTAN HCT to treat any other complaints unless your doctor or pharmacist tells you to
- Do not stop taking AVSARTAN HCT, or lower the dosage, without checking with your doctor
Things to be careful of
- If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
- Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
- The hydrochlorothiazide contained in this medicine could produce a positive analytical result in an antidoping test
Driving or taking machines
Be careful before you drive or use any machines or tools until you know how AVSARTAN HCT affects you.
As with many other medicines used to treat high blood pressure, AVSARTAN HCT, may cause dizziness or light-headedness in some people. If this occurs do not drive.
Make sure you know how you react to AVSARTAN HCT before you drive a car, operate machinery or do anything else that could be dangerous if you are dizzy or light-headed.
Drinking alcohol
Tell your doctor if you drink alcohol.
If you drink alcohol, dizziness or light-headedness may be worse.
Looking after your medicine
- Keep AVSARTAN HCT tablets in a cool dry place where the temperature stays below 25°C
- Keep your tablets in the blister pack until it is time to take them
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example:
- do not store it in the bathroom or near a sink, or
- do not store it in the car or on window sills.
Do not use this medicine after the expiry date.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. They are generally mild and do not normally require treatment to be interrupted. |
Serious side effects
| Serious side effects | What to do |
| Stop taking Avsartan HCT and you're your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. These are very serious side effects. You may need urgent medical attention or hospitalization. These side effects are very rare |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What AVSARTAN HCT contains
| Active ingredient (main ingredient) | Each AVSARTAN HCT 150/12.5 tablet contains 150 mg of irbesartan and 12.5 mg of hydrochlorothiazide |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | - |
| Active ingredient (main ingredient) | Each AVSARTAN HCT 300/12.5 tablet contains 300 mg of irbesartan and 12.5 mg of hydrochlorothiazide |
| Other ingredients (inactive ingredients) |
|
| Potential allergens | - |
| Active ingredient (main ingredient) | Each AVSARTAN HCT 300/25 tablet contains 300 mg of irbesartan and 25 mg of hydrochlorothiazide |
| Other ingredients (inactive ingredients) |
|
Do not take this medicine if you are allergic to any of these ingredients
What AVSARTAN HCT looks like
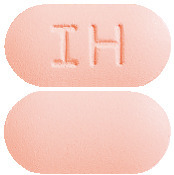
Avsartan HCT 150/12.5 mg tablets - Peach coloured, capsule shaped, biconvex, film-coated tablets, debossed with ‘IH’ on one face and plain on other face. Blister packs of 30 tablets. (Aust R 272655)
Avsartan HCT 300/12.5 mg tablets - Peach coloured, capsule shaped, biconvex, film-coated tablets, debossed with ‘IH2’ on one face and plain on other face.
Blister packs of 30 tablets. (Aust R 272659)
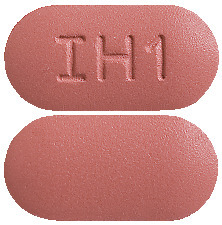
Avsartan HCT 300/25 mg tablets - Brick red coloured, capsule shaped, biconvex, film-coated tablets, debossed with ‘IH1’ on one face and plain on other face.
Blister packs of 30 tablets. (Aust R 272662)
Who distributes AVSARTAN HCT
AVSARTAN HCT is supplied in Australia by:
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
This leaflet was prepared in January 2024
Published by MIMS March 2024


 Adverse reactions (clinical events probably or possibly related to therapy as determined by the clinical investigator) that occurred in more than 2 hypertensive patients when they were taking irbesartan/hydrochlorothiazide and no additional study medications in premarketing clinical trials involving 2700 subjects, and that were not reported in the above tabulation of adverse events, are listed in the following section.
Adverse reactions (clinical events probably or possibly related to therapy as determined by the clinical investigator) that occurred in more than 2 hypertensive patients when they were taking irbesartan/hydrochlorothiazide and no additional study medications in premarketing clinical trials involving 2700 subjects, and that were not reported in the above tabulation of adverse events, are listed in the following section.

