What is in this leaflet
This leaflet answers some common questions about UREMIDE.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking UREMIDE against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What UREMIDE is used for
UREMIDE contains furosemide, which belongs to a family of medicines called diuretics. A diuretic helps reduce the amount of excess fluid in the body by increasing the amount of urine produced.
UREMIDE is used to treat swelling of the ankles, feet, legs, or even the brain or lungs. This swelling is called oedema and can occur in some heart, lung, liver or kidney conditions
UREMIDE may be used in some patients with more serious kidney problems who may have some fluid retention.
UREMIDE may also be used to lower high blood pressure (which is also called hypertension).
Everyone has blood pressure. This pressure helps move your blood around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension (high blood pressure) when your blood pressure stays higher than is needed, even when you are calm and relaxed.
If high blood pressure is not treated it can lead to serious health problems, including stroke, heart disease and kidney failure.
UREMIDE may be taken alone or in combination with other medicines to treat your condition.
Your doctor may have prescribed UREMIDE for another purpose.
Ask your doctor if you have any questions about why UREMIDE has been prescribed for you.
UREMIDE is only available with a doctor's prescription.
This medicine is not addictive.
Before you take UREMIDE
When you must not take it
Do not take UREMIDE if you are allergic to:
- furosemide
- medicines called sulfonamides (e.g. some types of antibiotics which are also referred to as 'sulfur antibiotics') or sulfonylureas which are medicines which can be used to treat diabetes.
- any of the ingredients listed at the end of this leaflet
Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not take UREMIDE if you have:
- certain liver and kidney problems
- no production or no passing of urine
- low blood pressure (hypotension)
- low sodium levels in your blood
- low potassium levels in your blood
- dehydration
- jaundice or history of jaundice in newborns or infants
- hepatic coma or precoma
Do not take this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy.
Do not take UREMIDE if you are breastfeeding or planning to breast-feed. The active ingredient, furosemide, passes into breast milk and there is a possibility your baby may be affected.
Do not take this medicine after the expiry date (Exp.) printed on the pack. If you take it after the expiry date has passed, it may not work as well.
Do not take UREMIDE if the packaging is torn or shows signs of tampering.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to:
- any of the ingredients listed at the end of this leaflet
- any other medicines called sulfonamides or sulfonylureas
- any other substances such as foods, preservatives or dyes.
Tell your doctor if you are pregnant or intend to become pregnant. Like most medicines of this kind, UREMIDE is not recommended to be used during pregnancy. If there is a need to consider UREMIDE during your pregnancy, your doctor will discuss the risks and benefits of taking it if you are pregnant.
Tell your doctor if you are breast-feeding or planning to breastfeed. UREMIDE passes into breast milk and there is a possibility your baby may be affected. Your doctor will discuss the risks and benefits of taking it if you are breast-feeding or planning to breast-feed.
Tell your doctor if you have or have had any medical conditions, especially the following:
- liver problems
- kidney problems
- heart problems
- high cholesterol levels
- asthma
- diabetes
- gout, a disease with painful, swollen joints
- passing less urine than is normal for you
- difficulty passing urine
- no production or no passing of urine
- prostate problems
- Systemic Lupus Erythematosus (SLE), a disease affecting the skin, joints and kidneys
Tell your doctor if you are on a salt restricted diet.
Restricting your salt intake may lead to increased side effects. If you have not told your doctor about any of the above, tell them before you start taking UREMIDE.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Some medicines should not be taken with UREMIDE. This includes large amounts of laxatives.
Some medicines may interfere with UREMIDE. These include:
- certain other fluid tablets or diuretic medicines
- medicines used to treat high blood pressure and some other heart conditions, especially ACE inhibitors or angiotensin receptor antagonists
- digoxin and other medicines used to treat heart failure
- non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin, medicines used to relieve pain, swelling and other symptoms of inflammation, including arthritis
- methotrexate, a medicine used to treat arthritis and some types of cancer
- probenecid, a medicine used to treat gout
- medicines used to relax muscles before or during surgery
- lithium, a medicine used to treat mood swings and some types of depression
- medicines used in emergency situations such as adrenaline (epinephrine) and noradrenaline (norepinephrine)
- cisplatin, a medicine used to treat cancer
- theophylline, a medicine used to treat asthma
- certain antibiotics, especially cephalosporins and aminoglycosides
- amphotericin B (amphotericin), a medicine used to treat fungal infections
- barbiturates, medicine used to treat epilepsy, to produce calmness, or to help you sleep
- narcotic/strong pain killers such as codeine and morphine
- insulin and medicines used to treat diabetes
- sucralfate, a medicine used to treat stomach ulcers
- anticonvulsant medicines such as chloral hydrate or phenytoin
- corticosteroids such as cortisone, prednisone or dexamethasone
- medicines used to treat thyroid conditions
- risperidone, an antipsychotic medication used to schizophrenia
- medicines used during scans to see the images of your body
These medicines may be affected by UREMIDE, or may affect how well it works. You may need to use different amounts of your medicine, or take different medicines. Your doctor or pharmacist will advise you.
You should not eat large amounts of liquorice when you are taking UREMIDE.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking UREMIDE.
How to take UREMIDE
How much to take
Follow the directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
Your doctor will tell you how many tablets you need to take each day and when to take them. This depends on your condition and how you respond to this medicine.
Ask your doctor or pharmacist if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, UREMIDE may not work as well and your problem may not improve.
How to take it
Swallow the tablets with a glass of water.
When to take it
UREMIDE is usually taken once or twice a day.
Take UREMIDE on an empty stomach. For example, 1 hour before food or 2 hours after food. Food can interfere with the absorption of UREMIDE.
Take UREMIDE at about the same time each day unless your doctor tells you otherwise. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take the medicine.
If your doctor prescribes UREMIDE to be taken once a day, it is best done in the morning, for example, before breakfast.
If you are taking UREMIDE more than once a day, take your first dose immediately before breakfast and take your last dose around 2:00 pm (on an empty stomach), unless your doctor tells you otherwise. UREMIDE may increase the amount of urine you pass, it will also increase the number of times you need to go to the toilet. By taking your last dose around 2:00 pm, there may be less chance that your sleep is disturbed.
How long to take it
Oedema
Continue taking your medicine for as long as your doctor tells you. The medicine helps control your condition and lowers the fluid build-up in your body.
Hypertension
Continue taking your medicine for as long as your doctor tells you. The medicine helps control your blood pressure, but it does not cure it. Continue taking the medicine until your doctor tells you to stop.
Ask your doctor or pharmacist if you are not sure how long to take the medicine for.
If you forget to take it
Do not try to make up for missed doses by taking more than one dose at a time. This may increase the chance of getting an unwanted side effect.
If it is almost time for your next dose, skip the dose you missed and take the next dose when you are meant to.
If there is still a long time to go before your next dose, take the missed dose as soon as you remember, and then go back to taking it as you would normally.
If you are not sure what to do, ask your doctor or pharmacist.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much UREMIDE.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much UREMIDE, you may feel confused, dehydrated, dizzy or you may pass excessive urine.
While you are taking UREMIDE
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking this medicine.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking UREMIDE.
If you plan to have a surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking UREMIDE. Your blood pressure may drop suddenly.
If you become pregnant while you are taking this medicine, tell your doctor or pharmacist immediately.
Tell your doctor if you have excessive vomiting or diarrhoea while taking UREMIDE or if you experience any of the following symptoms:
- dry mouth or thirst
- fainting
- weakness, tiredness or drowsiness
- muscle pain or cramps
- passing less urine than normal
- fast heart beat
If you experience these symptoms, you may be dehydrated because you are losing too much water.
Make sure you drink enough water during any exercise and during hot weather when you are taking UREMIDE, especially if you sweat a lot. If you do not drink enough water while taking UREMIDE, you may feel faint or lightheaded or sick. This is because your blood pressure is dropping suddenly and you are becoming dehydrated. If you continue to feel unwell, tell your doctor.
If you are about to have any blood tests, tell your doctor that you are taking UREMIDE. There may be some interference with the results of these tests.
If you are taking UREMIDE to treat high blood pressure, make sure you have your blood pressure checked when your doctor says to make sure UREMIDE is working properly.
Things you must not do
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not use this medicine to treat any other complaints unless your doctor tells you to.
Do not stop taking your medicine, or change the dosage, without checking with your doctor.
Things to be careful of
If you feel light-headed, dizzy or faint, get up slowly when getting out of bed or standing up. You may feel light-headed or dizzy when you begin to take UREMIDE. This is because your blood pressure is falling suddenly. Standing up slowly, especially when you get up from beds or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Be careful driving or operating machinery until you know how UREMIDE affects you. Diuretic medicines may cause dizziness or light-headedness in some people. Make sure you know how you react to your medicine before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy or lightheaded.
If this occurs do not drive.
If you drink alcohol or take strong painkillers, dizziness or light-headedness may be worse.
The effects of alcohol could be made worse while taking UREMIDE. It is not recommended that you drink alcohol while taking UREMIDE.
If you are taking UREMIDE for a long period of time, you should check with your doctor to determine whether or not you should eat more potassium-containing foods or take potassium supplements. However, increasing the amount of potassium in your diet may not be necessary and could be harmful. Check with your doctor.
UREMIDE may cause your skin to become more sensitive to the sun. If this happens you should take care to wear protective clothing including a hat and sun block when you are outside.
Things that may help your condition
Some self-help measures suggested below may help your condition.
- alcohol - your doctor may advise you to limit your alcohol intake
- diet - eat a healthy diet which includes plenty of fresh vegetables, fruit, bread, cereals and fish. Also eat less fat and sugar
- exercise - regular exercise helps reduce blood pressure and helps the +heart get fitter, but it is important not to overdo it. Walking is a good exercise, but try to find a route that is fairly flat. Before starting any exercise, ask your doctor about the best kind of program for you
- salt - if you have high blood pressure, your doctor may advise you to watch the amount of salt in your diet. To reduce your salt intake you should avoid using salt in cooking or at the table
- smoking - your doctor may advise you to stop smoking or at least to cut it down
- weight - your doctor may suggest that you lose some weight to help lower your blood pressure and help lessen the amount of work your heart has to do. Some people may need a dietician's help to lose weight.
Talk to your doctor or pharmacist about these measures and for more information.
Side effects
All medicines have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking UREMIDE.
UREMIDE helps most people with high blood pressure or fluid retention, but it may have unwanted side effects in a few people.
Tell your doctor if you notice any of the following and they worry you:
- very dry mouth or unusual thirst
- weight loss
- weakness or tiredness
- numbness or tingling in the hands and/or feet
- calf muscle spasms
- muscle pains or cramps
- restlessness
- drowsiness or a lack of energy
- dizziness or light-headedness
- headache
- fever
- vomiting or nausea
- diarrhoea
- blurred or impaired vision
- unusual bleeding or bruising under the skin
- ringing or buzzing in the ears
- confusion
These are more common side effects of UREMIDE. Mostly they are mild or short-lived.
Tell your doctor as soon as possible if you notice any of the following:
- irregular or fast heart beat
- gout, a disease with painful, swollen joints
- severe dizziness or a spinning sensation
- severe stomach pain, often with nausea and vomiting
- flaking or peeling of the skin
- passing less urine than is normal for you
- frequent infections such as fever, severe chills, sore throat or mouth ulcers
- bruising or bleeding more easily than normal, nose bleeds
- symptoms of anaemia such as tiredness, being short of breath when exercising, dizziness and looking pale
- increased sensitivity to sunlight
- loss of control of your bladder or bowels (incontinence)
- deafness or ringing in the ears
These may be serious side effects of UREMIDE. You may need urgent medical attention. Serious side effects are uncommon.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- sudden signs of allergy such as rash, itching or hives (pinkish, itchy raised areas) on the skin, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or trouble breathing
- chest pain
- fainting or having a rapid, weak pulse
- lockjaw
- red, often itchy spots similar to the rash seen with measles which starts on the limbs and sometimes on the face and body. The spots may blister and may progress to form raised red, pale-centred marks. Those affected may have fever, sore throat, headache with or without diarrhoea
- yellowing of the skin and/or eyes (jaundice)
These are serious side effects. You may need urgent medical attention or hospitalisation. These side effects are very rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some people.
Ask your doctor or pharmacist to answer any questions you may have.
After taking UREMIDE
If you have any queries about any aspect of your medicine, or any questions regarding the information in this leaflet, discuss them with your doctor or pharmacist.
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store UREMIDE or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking UREMIDE, or the medicine has passed its expiry date, ask your pharmacist what to do with any that is left over.
Return any unused medicine to your pharmacist.
Product description
What it looks like
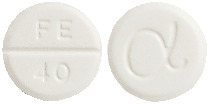
UREMIDE is a 8 mm flat bevelled edged white tablet marked FE/40 on one side, alpha symbol on the reverse.
Each pack contains 30 or 100 tablets.
Ingredients
The active ingredient in UREMIDE is furosemide. Each UREMIDE tablet contains 40 mg of furosemide.
The tablets also contain:
- lactose monohydrate
- maize starch
- pregelatinised maize starch
- magnesium stearate.
UREMIDE tablets contain sugars as lactose and trace quantities of sulfites.
Manufacturer
UREMIDE is made in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in September 2023.
Australian registration numbers:
AUST R 17704
UREMIDE® is a Viatris company trade mark
UREMIDE_cmi\Sep23/00
Published by MIMS October 2023

 Molecular formula: C12H11ClN2O5S. Molecular weight: 330.74.
Molecular formula: C12H11ClN2O5S. Molecular weight: 330.74.