Practice Review – Rheumatoid arthritis
Australian rheumatologists and selected immunologists have received PBS Practice Reviews (Oct 2020 and May 2022) on their prescribing of medicines for rheumatoid arthritis. Find out more about how to read your Practice Review.
Quality use of b/tsDMARDs and other medicines
Australian rheumatologists and selected immunologists recently received a PBS Practice Review titled ‘Rheumatoid arthritis: Quality use of b/tsDMARDs and other medicines’, developed by NPS MedicineWise in collaboration with the Australian Rheumatology Association (ARA).
The Practice Review is intended to help clinicians reflect on their prescribing of biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) and other medicines for rheumatoid arthritis (RA).
The information on this page may help prescribers interpret their individual PBS Practice Review data. National and aggregate state data are also included below.
Download and print a sample report
Frequently asked questions
Continuing professional development
For RACP Fellows, the time you spend using the ‘Quality use of b/tsDMARDs and other medicines’ Practice Review report to review and adjust your practice meets the requirements for CPD under Category 3 – Measuring Outcomes.
Use this CPD Shortcut to record this activity in MyCPD. Some fields have been pre-populated for your convenience; please adjust these as necessary before saving the activity.
Aggregate state and national data
Use of csDMARDs before starting b/tsDMARD treatment
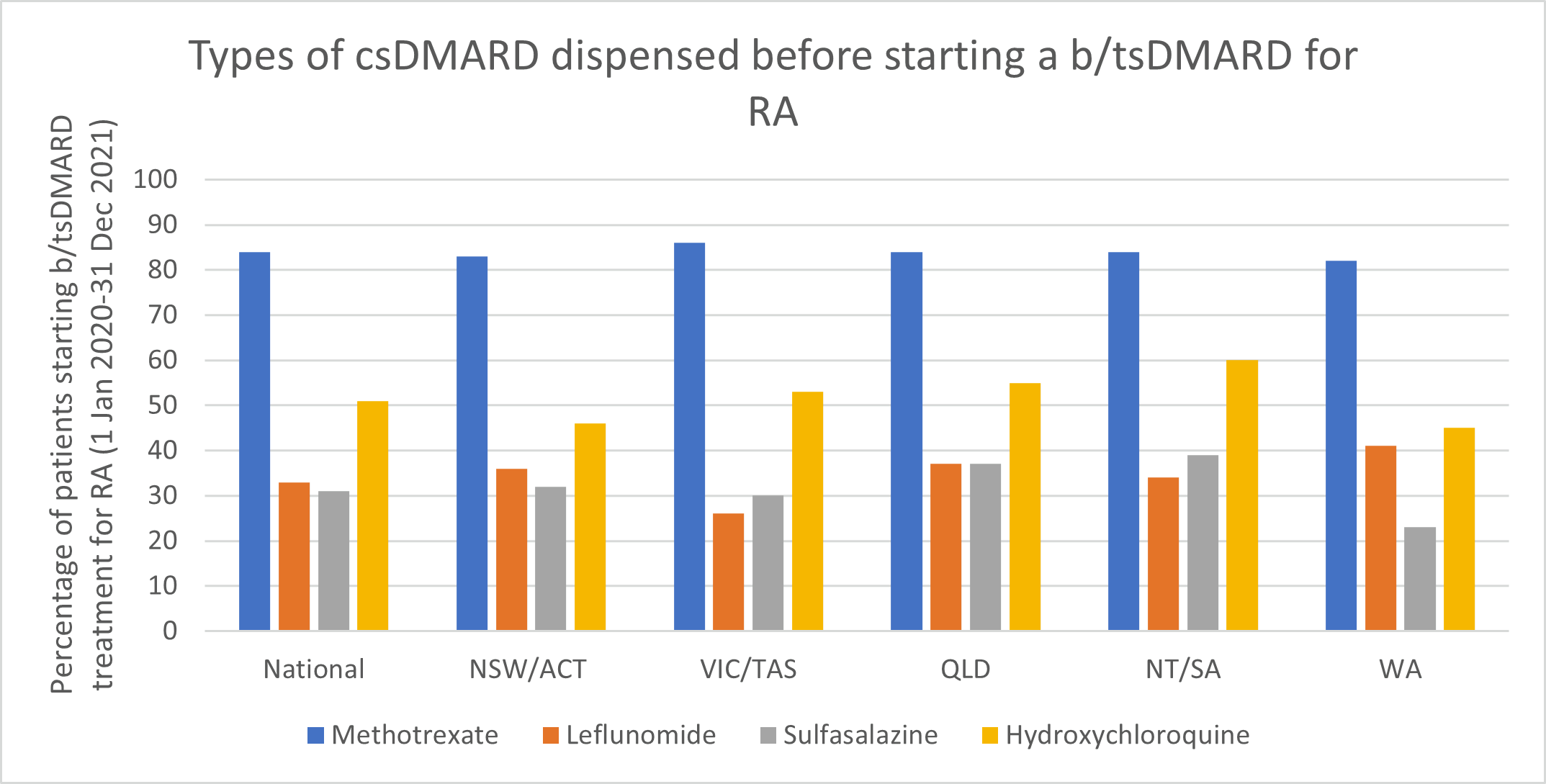
- Methotrexate (MTX) remains the first-line DMARD for rheumatoid arthritis (RA).
- Consider using MTX in combination with other DMARDs as initial therapy in people with rheumatoid arthritis.
Types of MTX used before starting a b/tsDMARD for RA, 2019–2021
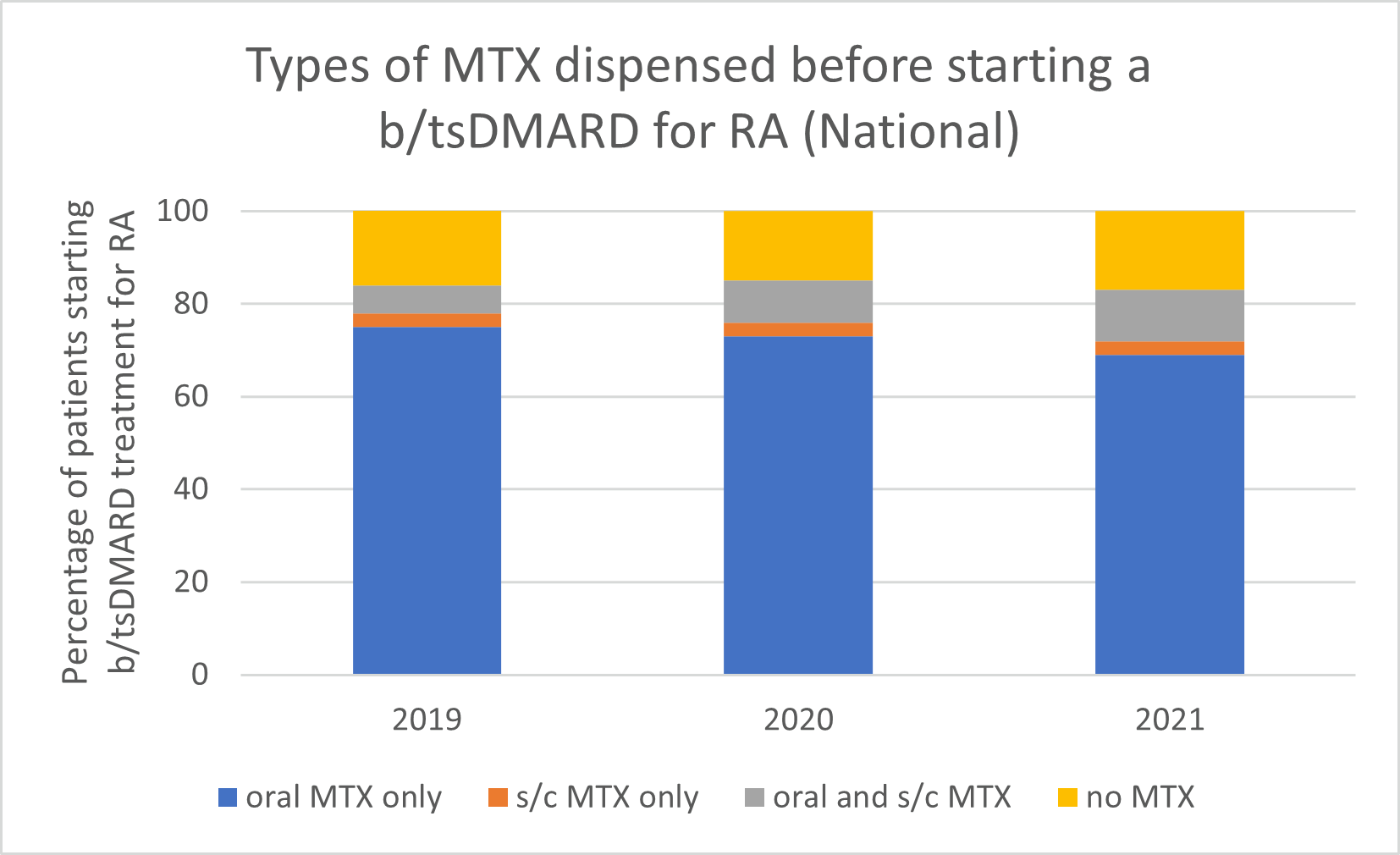
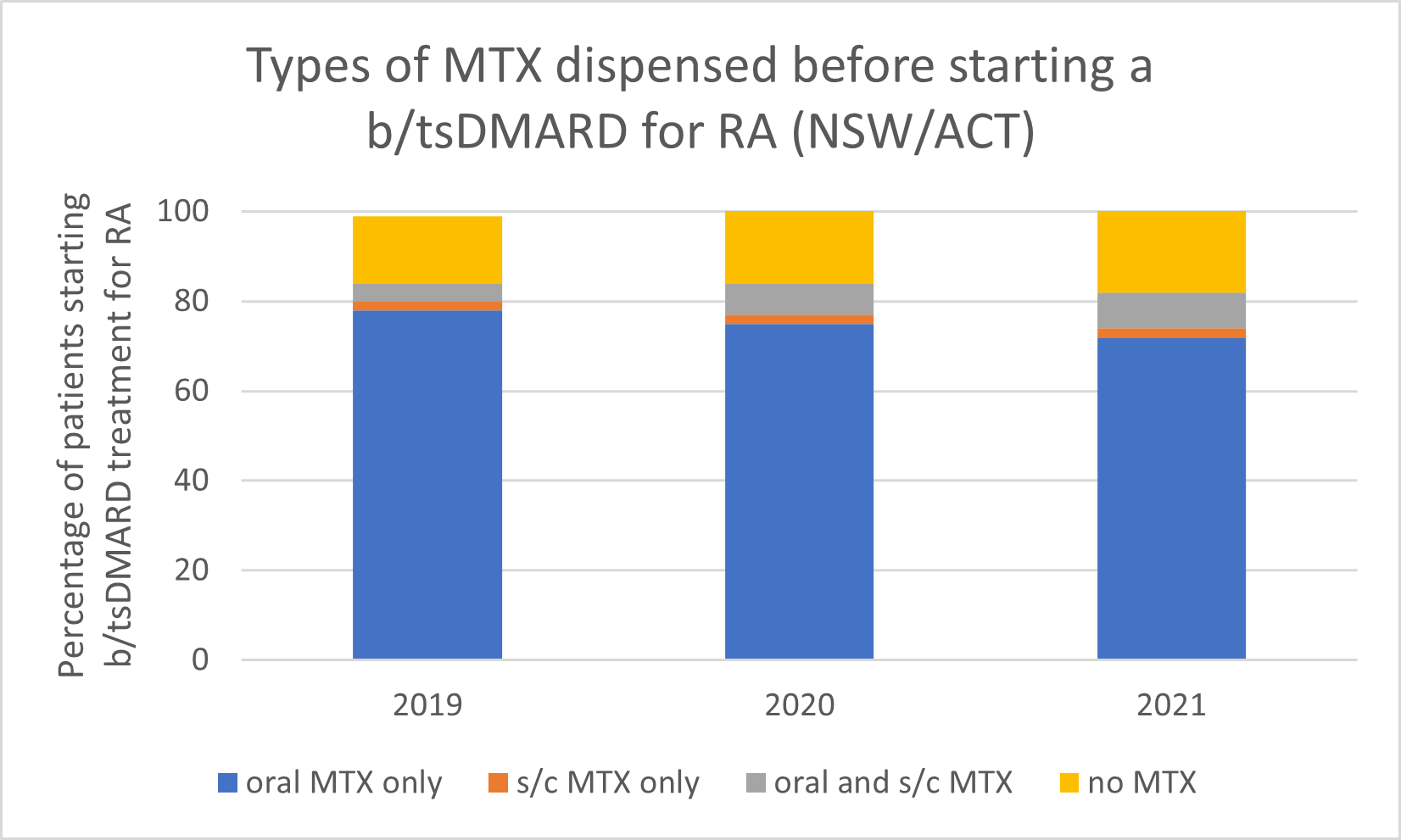
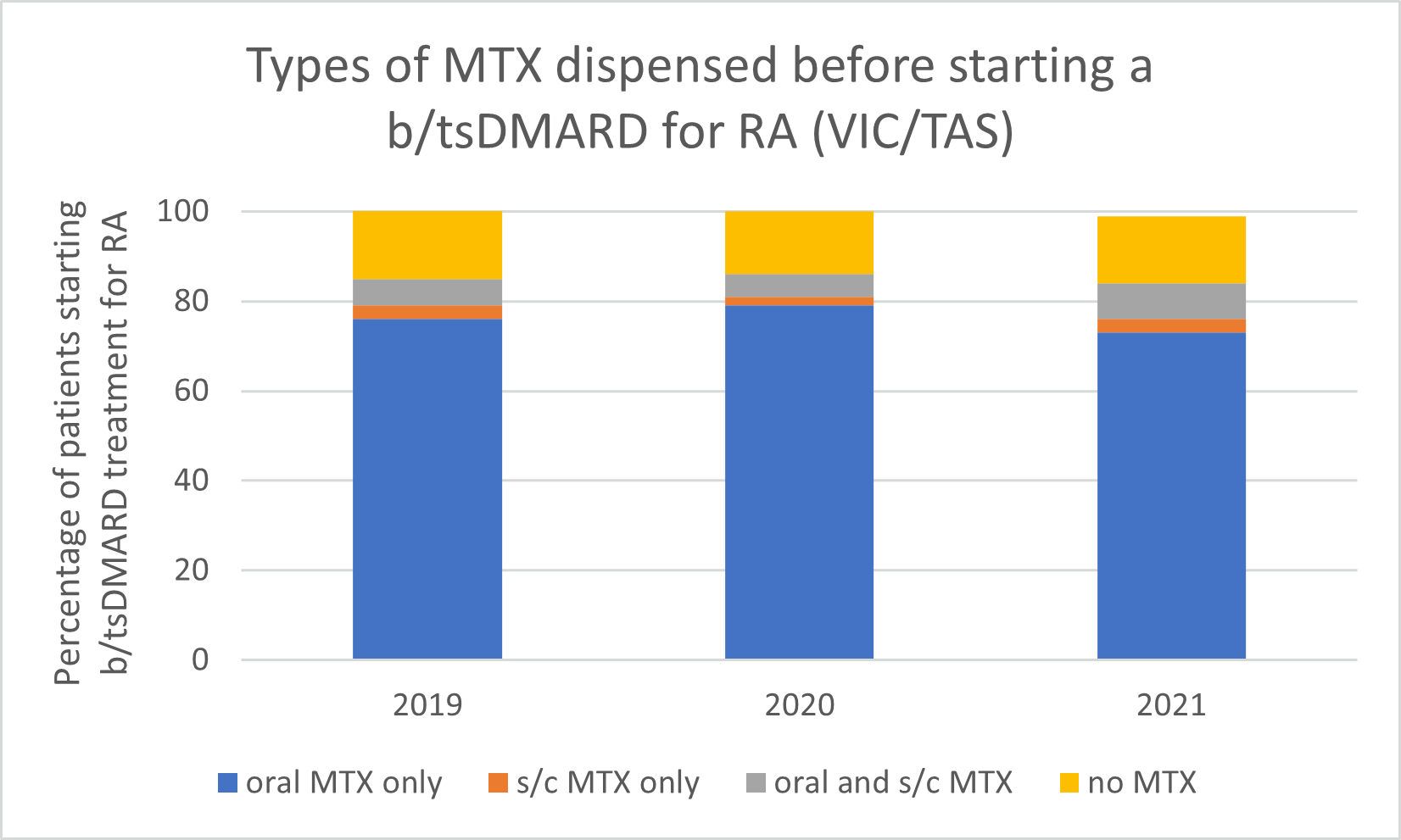
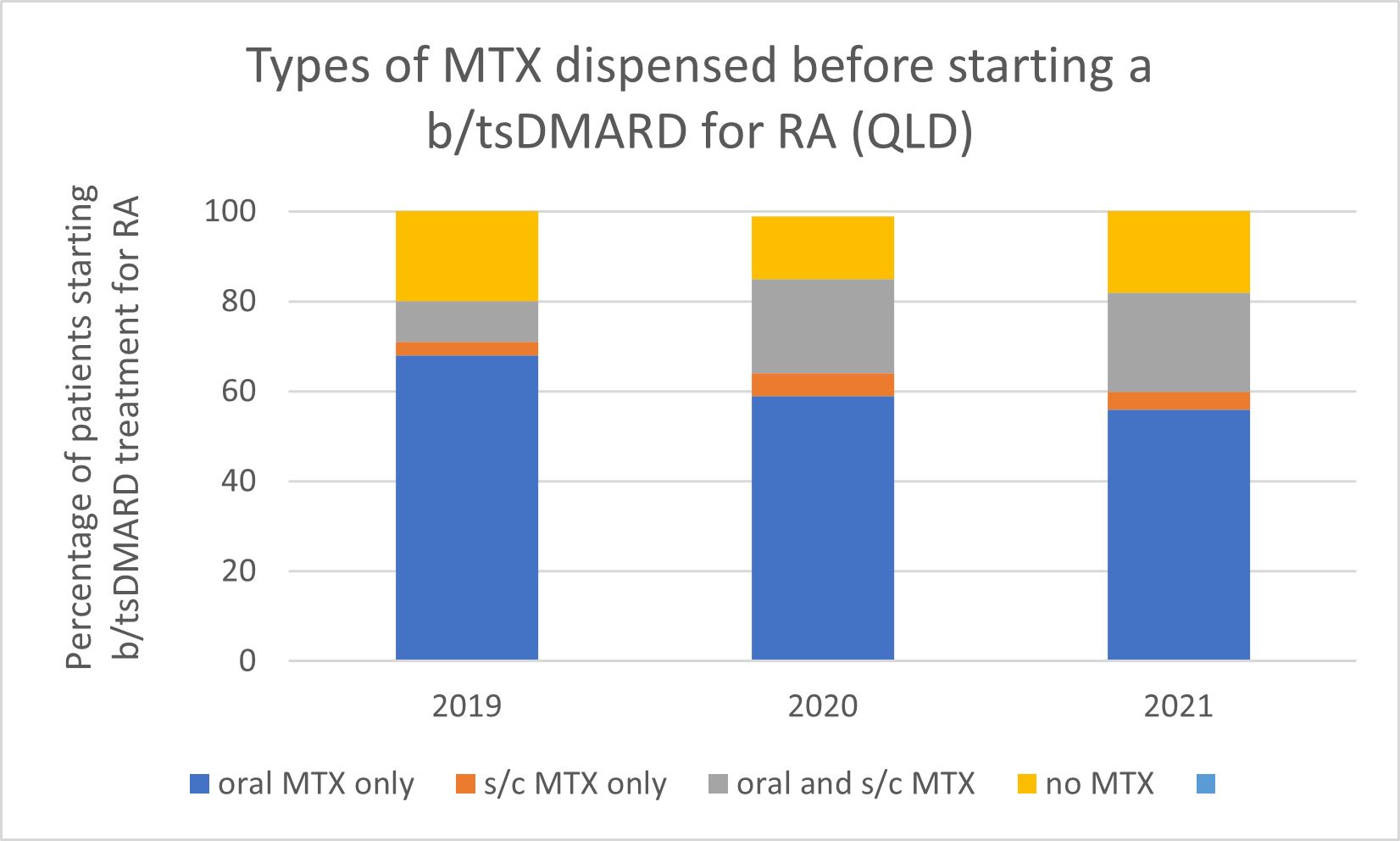
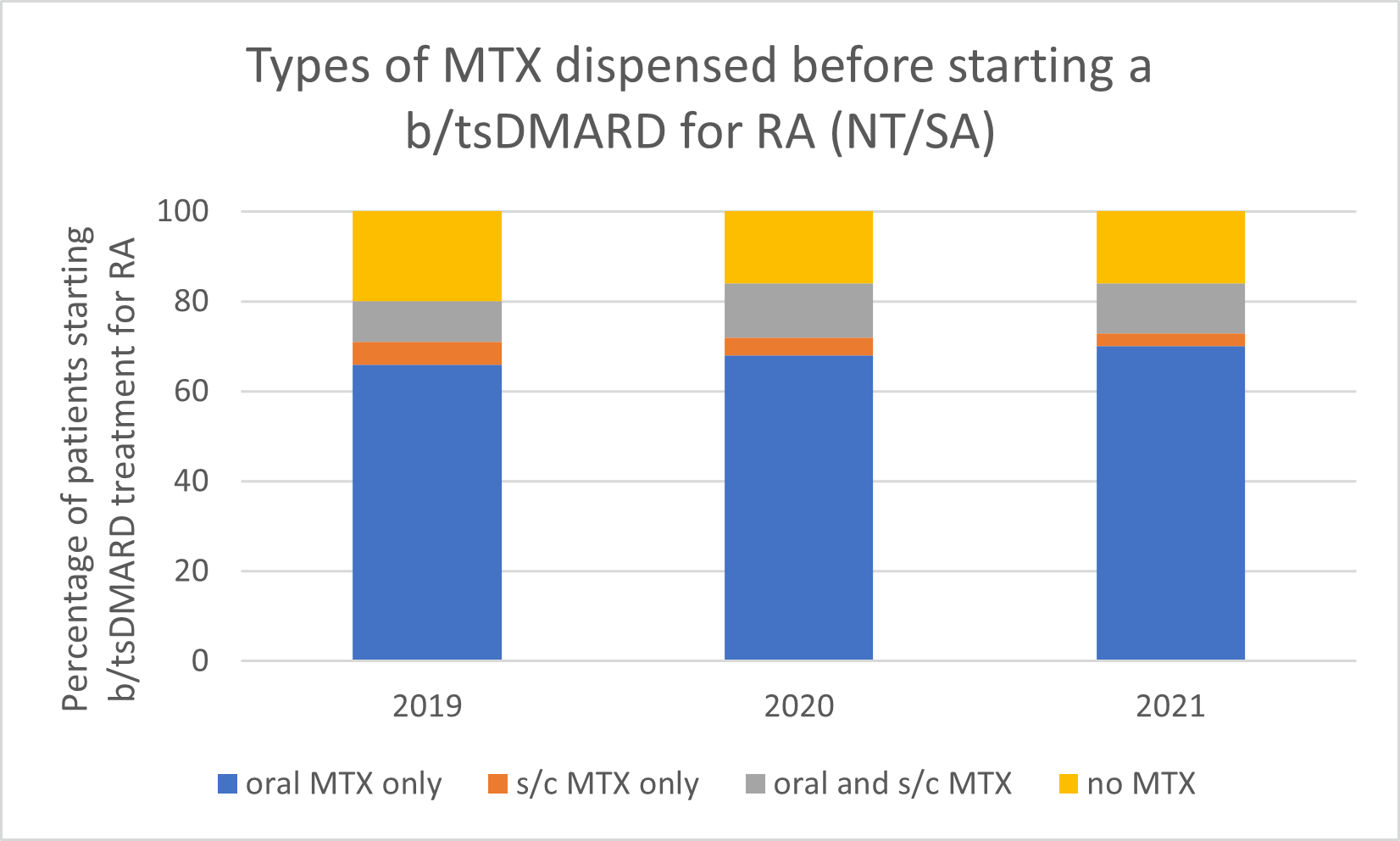
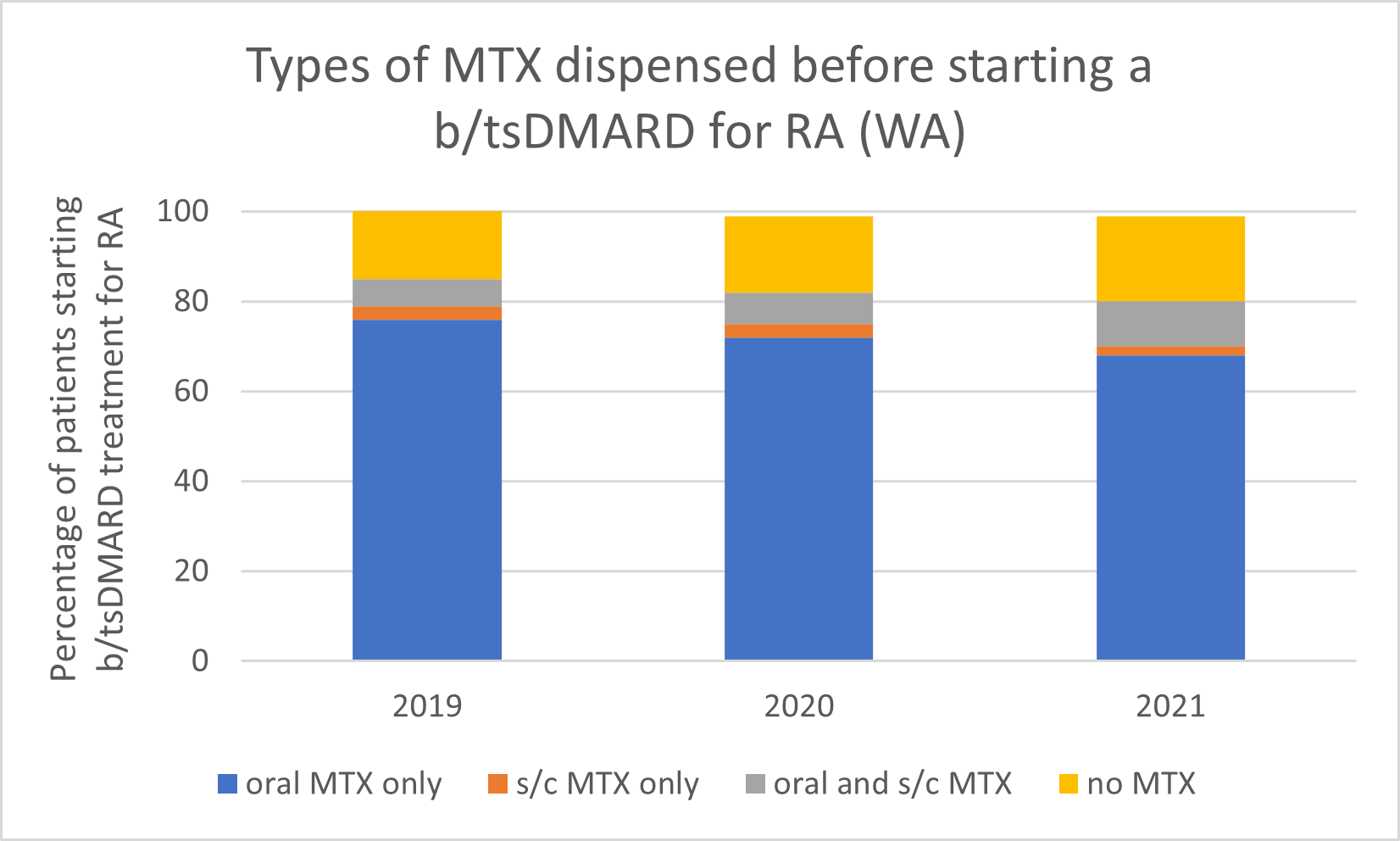
Note: Because of rounding, percentages may range from 99% to 101%.
- Subcutaneous MTX is more efficacious and generally better tolerated than oral MTX, with significantly fewer associated gastrointestinal (GI) adverse effects (eg, nausea and diarrhoea).
- Switching from oral to subcutaneous MTX is highly effective; in a study, 72% of patients switched remained on subcutaneous MTX for the duration of the 5-year observational period without the addition of a biologic.
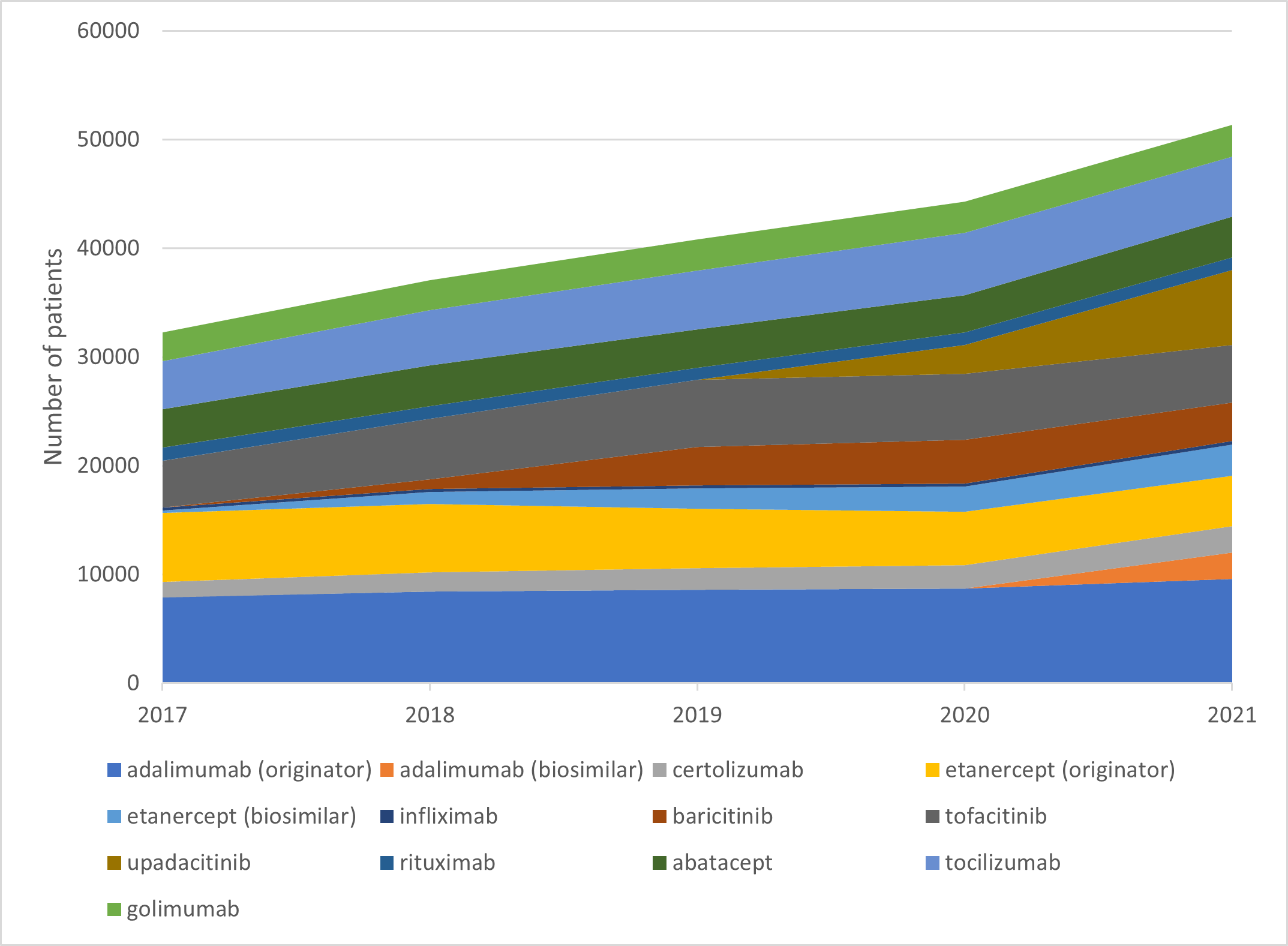
Choice of biologic when starting b/tsDMARD treatment
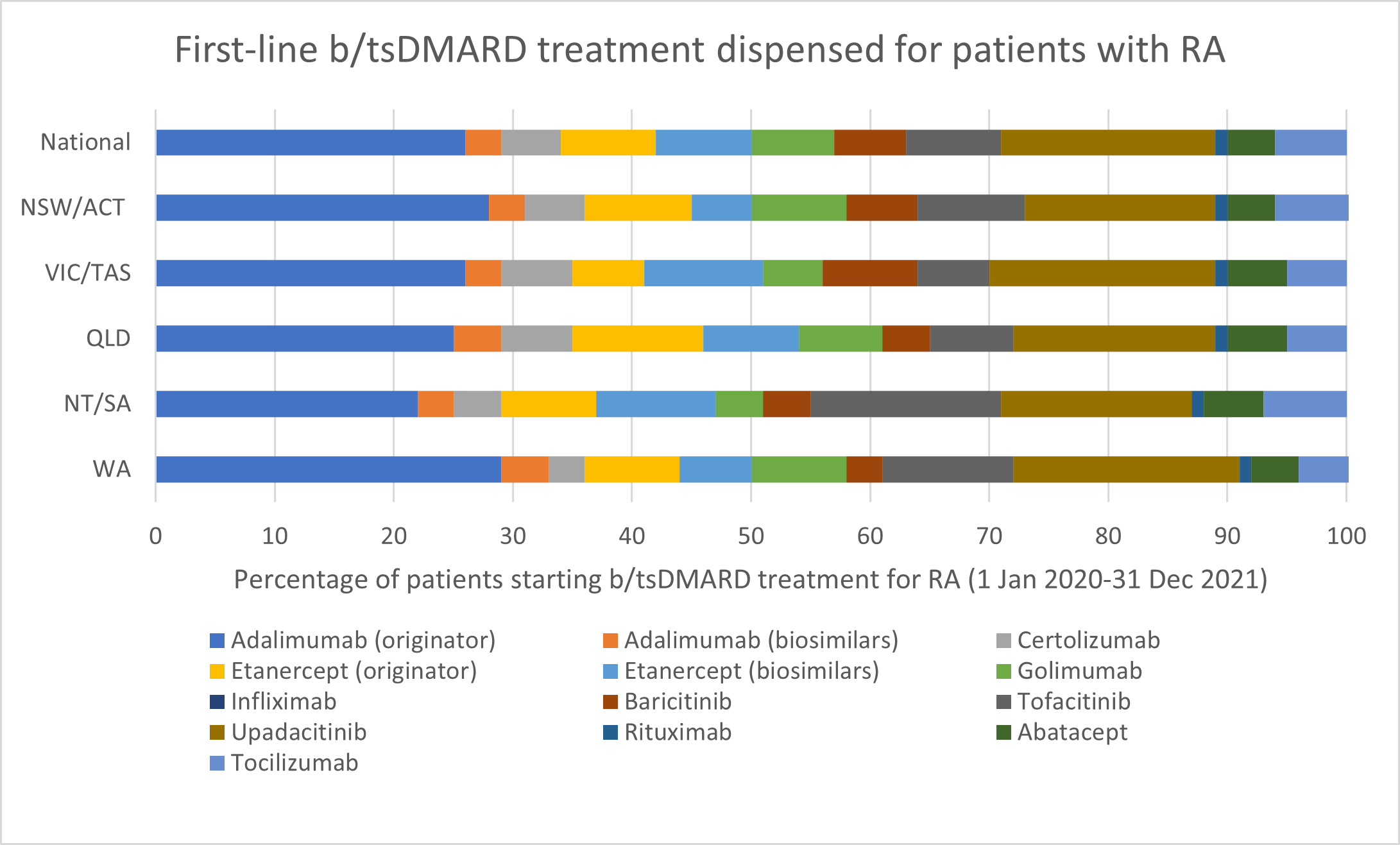
b/tsDMARDs are generally considered to have comparable efficacy for RA. Individualise choice of b/tsDMARD.
Number of unique patients dispensed prescriptions for b/tsDMARDs for RA (Australia wide, 2017–21)
Glucocorticoid use
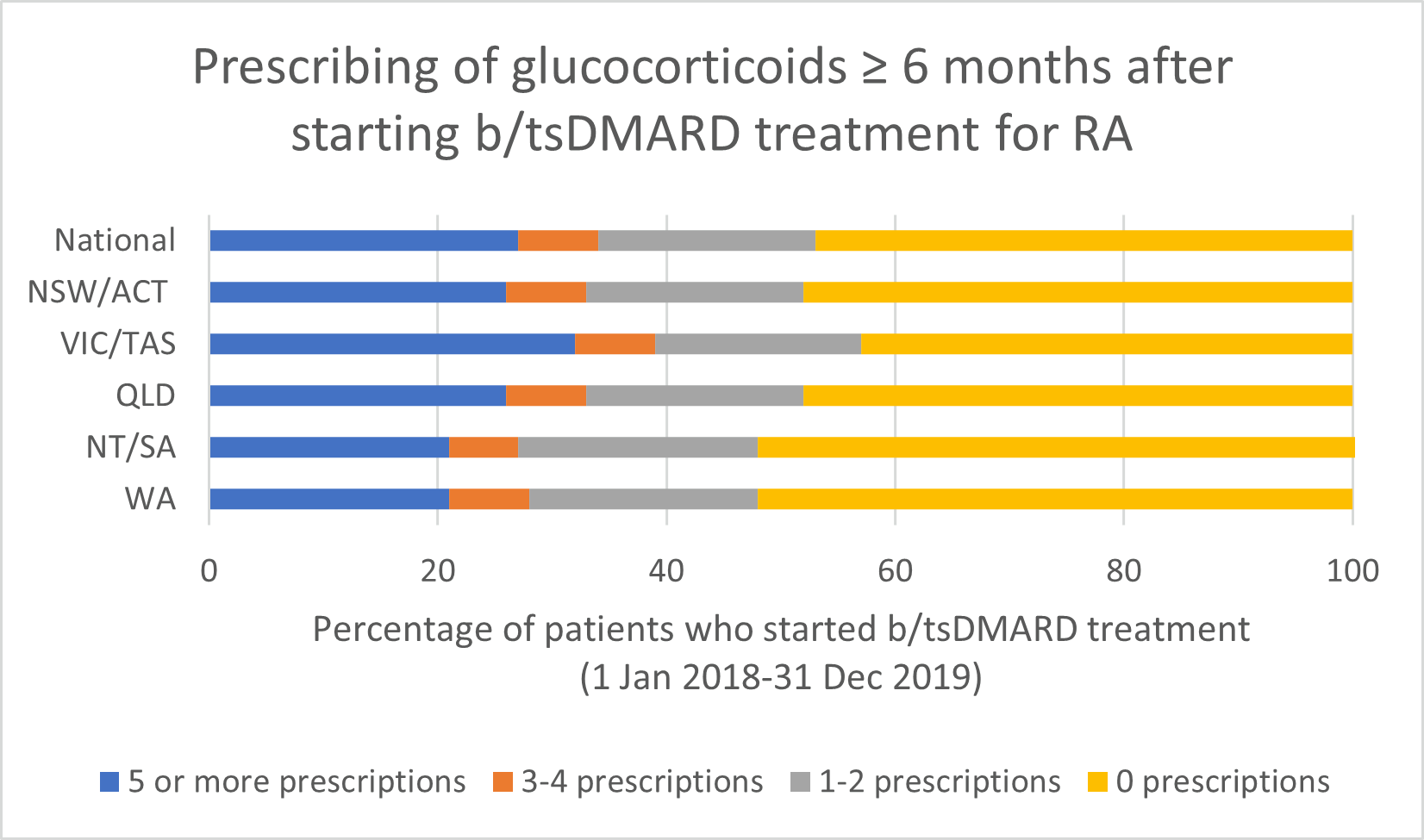
Note: Because of rounding, percentages may range from 99% to 101%.
- If a glucocorticoid is considered necessary, guidelines recommend short-term use to control flare-ups and limiting use to ≤ 3 months for bridging therapy when starting or changing DMARDs.
Opioid use
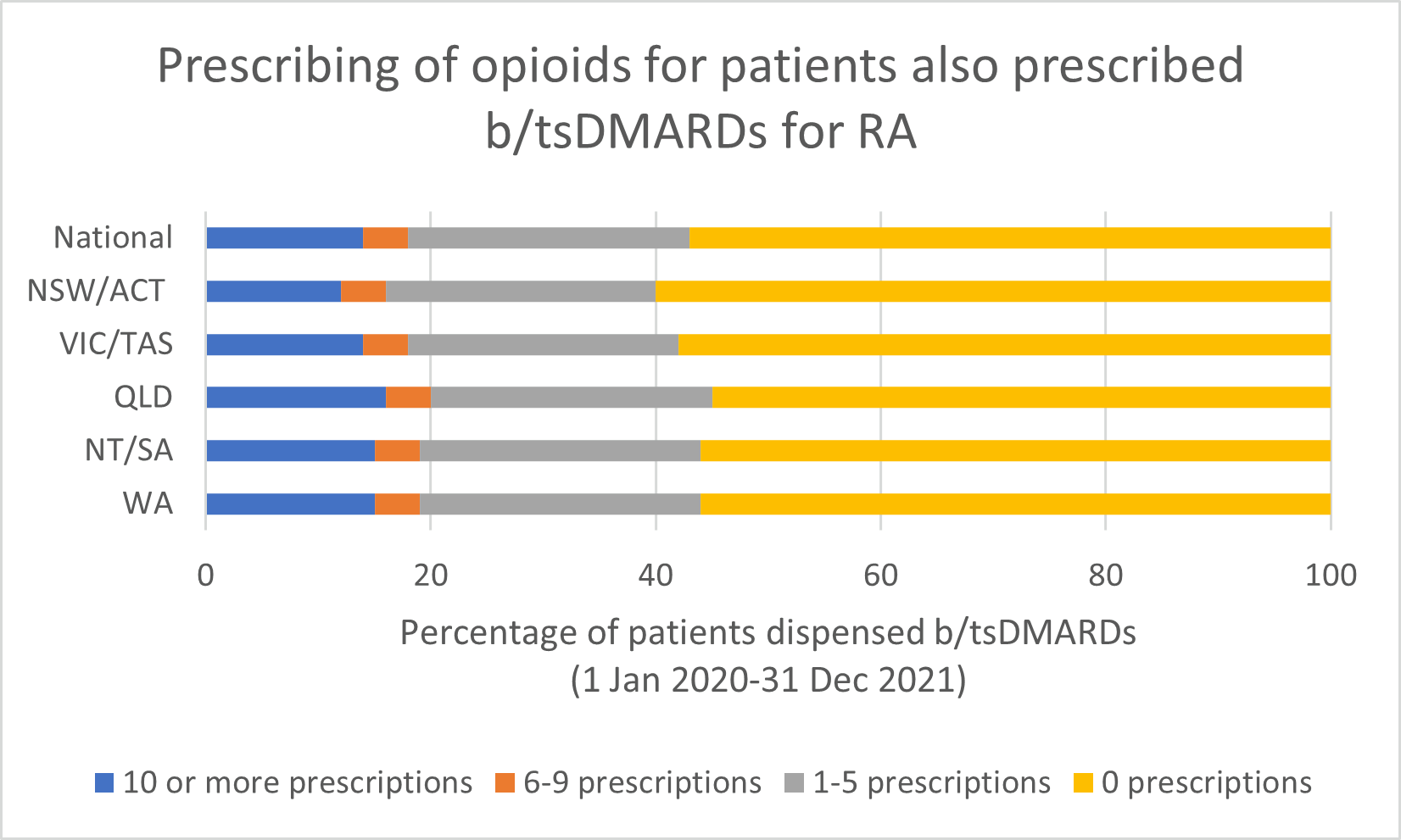
Note: Because of rounding, percentages may range from 99% to 101%.
- There is insufficient evidence to support the long-term use of opioids for pain management by patients with RA.
- Any benefits of opioids can usually be determined within a 2–4 week trial of opioid treatment. Short-term use (< 6 weeks) of weak opioids may provide some analgesia, but adverse effects and the risk of harms (including increased risk of serious infection and risk of overdose) limit their use.
Additional resources
Acknowledgments and contributions
These PBS Practice Reviews have been developed in collaboration with:
- Australian Rheumatology Association (ARA) representatives: Prof Catherine Hill and Dr Claire Barrett.
- Rheumatologist advisor: Dr Premarani Sinnathurai.
- Rheumatologists engaged in user testing and reviews: Dr Robert Baume, Dr Helen Cooley, Dr Narainraj Kamalaraj, Dr David Liew, Dr Daman Langguth, Dr Ingrid Hutton, Dr Jenny Walker and Dr Gemma Winkler.
- Targeted Therapies Alliance (ARA, Cochrane MSK, NPS MedicineWise and University of South Australia).

Practice Review references
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020.
- Therapeutic Guidelines. West Melbourne: Therapeutic Guidelines Ltd (accessed 26 April 2022).
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res 2021;73:924-39.
- ANZMUSC. An Australian Living Guideline for the Pharmacological Management of Inflammatory Arthritis. 2022.
- Rohr MK, Mikuls TR, Cohen SB, et al. Underuse of Methotrexate in the Treatment of Rheumatoid Arthritis: A National Analysis of Prescribing Practices in the US. Arthritis Care Res (Hoboken) 2017;69:794-800.
- Tanaka E, Kawahito Y, Kohno M, et al. Systematic review and meta-analysis of biosimilar for the treatment of rheumatoid arthritis informing the 2020 update of the Japan College of Rheumatology clinical practice guidelines for the management of rheumatoid arthritis. Mod Rheumatol 2021;32(1):74-86.
- Kay J, Schoels MM, Dörner T, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis 2018;77(2):165-74.
- US Food and Drug Administration. Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz, Xeljanz XR (tofacitinib). 2021. https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis?utm_medium=email&utm_source=govdelivery
(accessed 18 October 2021). - Therapeutic Goods Administration. Shortages of tocilizumab (Actemra) medicines. 2021. https://www.tga.gov.au/alert/shortages-tocilizumab-actemra-medicines (accessed 18 October 2021).
